Sequencing Algae’s Genome May Aid Biofuel Production
University of Washington scientists have sequenced the complete genetic makeup of an important algae
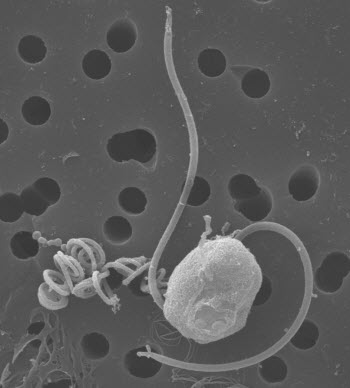 Close-up view of Chrysochromulina tobin.Image credit: Rose Ann CattolicoThere’s an ancient group of algae that evolved in the world’s oceans before our backboned ancestors crawled onto land. They are so numerous that their gigantic blooms can affect the weather, and they account for 30 to 40 percent of all photosynthesis in the world’s oceans.
Close-up view of Chrysochromulina tobin.Image credit: Rose Ann CattolicoThere’s an ancient group of algae that evolved in the world’s oceans before our backboned ancestors crawled onto land. They are so numerous that their gigantic blooms can affect the weather, and they account for 30 to 40 percent of all photosynthesis in the world’s oceans.
But until recently, scientists interested in these single-celled creatures knew next to nothing about their genes.
University of Washington scientists have sequenced the complete genetic makeup of one of these algae. As they recently reported in the journal PLOS Genetics, it is only the second time that researchers have sequenced the genome of one of these ecologically important and plentiful algae, known as haptophytes. Researchers hope to better understand haptophytes and perhaps transform them into an important new tool for aquaculture, biofuel production and nutrition.
“Haptophytes are really important in carbon dioxide management and they form a critical link in the aquatic foodchain,” said senior author and UW biology professor Rose Ann Cattolico. “This new genome shows us so much about this group.”
Related article: Algae to Crude Oil: Million-year Natural Process Takes Minutes in the Lab
The haptophyte Cattolico and her team studied is Chrysochromulina tobin, and it thrives in oceans across the globe. The researchers spent years on a series of experiments to sequence all of Chrysochromulina‘s genes and understand how this creature turns different genes on and off throughout the day. In the process, they discovered that Chrysochromulina would make an ideal subject for investigating how algae make fat, a process important for nutrition, ecology and biofuel production.
“It turns out that their fat content gets high during the day and goes down during the night,” said Cattolico. “A very simple pattern, and ideal for follow-up.”
She believes that that these extreme changes in fat content — even within the span of a single day — may help ecologists understand when microscopic animals in the water column choose to feast upon these algae. But knowledge of how the algal species regulates its fat stores could also help humans.
“Algae recently became more familiar to the general populace because of biofuel production,” said Cattolico. “We needed a simple alga for looking at fat production and fat regulation.”
This led Cattolico to team up with Blake Hovde, then a graduate student in the UW Department of Genome Sciences, to sequence the complete genome of this species. Hovde wanted to work on algae in biofuel production, and Chrysochromulina was ideally suited for the task because, unlike most other haptophytes, it has no protective cell wall.
Related article: Algae from Clogged Waterways Could Serve as Biofuels and Fertilizer
Hovde and Cattolico uncovered other surprises in the Chrysochromulina genome. Like other algae and plants, Chrysochromulina uses light to make food, through the process of photosynthesis. But they also found another gene, called xanthorhodopsin, that may let the alga harvest light and do work outside of the traditional photosynthesis pathway. Cattolico does not know how the alga uses this gene, but would like to investigate this in the future.
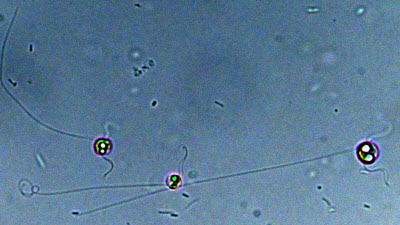 Close-up view of Chrysochromulina tobin.Image credit: Rose Ann CattolicoIn addition, they identified numerous genes that appear to harbor antibiotic activity, which may be useful as the need for new antibiotics continues to rise. But Chrysochromulina is not universally against bacteria. Through this project, Cattolico and her team discovered that there are at least 10 bacterial species that appear to enjoy living near Chrysochromulina.
Close-up view of Chrysochromulina tobin.Image credit: Rose Ann CattolicoIn addition, they identified numerous genes that appear to harbor antibiotic activity, which may be useful as the need for new antibiotics continues to rise. But Chrysochromulina is not universally against bacteria. Through this project, Cattolico and her team discovered that there are at least 10 bacterial species that appear to enjoy living near Chrysochromulina.
“That leads to some interesting questions,” said Cattolico. “Is Chrysochromulina selectively using its antimicrobials? Is it ‘farming’ beneficial bacteria in its neighborhood?”
Cattolico would like to understand how these bacteria affect which genes Chrysochromulinaswitches on and off. That information may pave the way for new studies of the ecology of haptophytes, which could be critical in the face of a changing global climate.
Related article: Advancing Algae’s Viability as a Biofuel
“Haptophytes are very important to our ocean health, especially with these massive —sometimes toxic — blooms they make,” said Cattolico. “We need to understand this issue because ecosystems are only going to get more compromised with climate change.”
The research was published Sept. 23 in the online, open-access journal PLOS Genetics. First author Hovde is now a postdoctoral researcher at the Los Alamos National Laboratory. Other UW co-authors are Chloe Deodato, Heather Hunsperger, Scott Ryken, Will Yost, Johnathan Patterson and Ray Monnat. Ramesh Jha and Shawn Starkenburg were co-authors from Los Alamos National Laboratory, as well as Steven Barlow from San Diego State University. The research was funded by the U.S. Department of Energy, Washington Sea Grant, the National Science Foundation, the National Institutes of Health, Los Alamos National Laboratory and the Defense Threat Reduction Agency.
