Making the Business Case: Preparing Exact Concentrations in Just the Right Quantities
Arguing against lab automation is becoming more difficult, particularly for sample prep. With reagents and solvents costing what they do, a business case based on direct cost savings for critical materials stands on its own.
Arguing against lab automation is becoming more difficult, particularly for sample prep. With reagents and solvents costing what they do, a business case based on direct cost savings for critical materials stands on its own.
Conventional sample preparation generates on a cost or weight basis much more waste than product. Mettler Toledo estimates that more than 99 percent of prepared solutions are never used and that manual prep of samples and standards for analytical methods accounts for up to 82 percent of a lab’s solvent usage, 61 percent of labor time, and 49 percent of outof- specification (OOS) errors. Moreover, continuing improvements in data management capabilities—the second-leading time killer in a lab, at 27 percent—will have the effect of increasing the share for sample preparation in nonautomated laboratories.
“Manual handling always entails the risk of process failure and unacceptable variability from user to user,” says Dr. Carsten Buhlmann, international product manager for automation at Eppendorf (Hamburg, Germany).
 Liquid Handling System / Encore Multispan / Agilent Technologies / www.agilent.com The most obvious effects of OOS errors are rework and time wasted in the (often vain) attempt to identify, remediate, and report on the root cause of the error. Rework, arguably a lab manager’s worst nightmare, involves redeploying personnel, instrumentation, reagents, etc., to a job that should have been completed the first time and diverting those resources from new work. According to Mettler Toledo, OOS results cost between $3,000 and $10,000 and can shut down critical lab functions for anywhere from three days to several weeks, resulting in serious loss of revenue and/or productivity.
Liquid Handling System / Encore Multispan / Agilent Technologies / www.agilent.com The most obvious effects of OOS errors are rework and time wasted in the (often vain) attempt to identify, remediate, and report on the root cause of the error. Rework, arguably a lab manager’s worst nightmare, involves redeploying personnel, instrumentation, reagents, etc., to a job that should have been completed the first time and diverting those resources from new work. According to Mettler Toledo, OOS results cost between $3,000 and $10,000 and can shut down critical lab functions for anywhere from three days to several weeks, resulting in serious loss of revenue and/or productivity.
Laboratories with the capability of taking a lean/six sigma approach to OOSs go through the normal drill of analyzing and identifying waste and variability. This approach quickly leads to the observation that the more human steps involved in a process, the more likely the incursion of systematic and nonsystematic errors.
According to Dr. Charles Ray, former associate director of analytical R&D at Bristol-Myers Squibb and currently a consultant at CWR Consulting, a formal lean program is often not necessary to resolve OOS incidents. “A team can simply sit down and go through a very detailed workflow chart and highlight the problem areas. These may be steps that no longer make sense, that have a high likelihood of involving both determinate and indeterminate errors, and where efficiencies and cost reductions can occur.”
Gravimetric (weighing) automation is becoming an acceptable strategy for avoiding overprep or inaccurately formulated solutions, yet most systems remain unautomated. Automated balances still require operators to add and remove vials. Their main benefits, as noted by Joanne Ratcliff, Ph.D., an analytical chemist and current communications project manager at Mettler Toledo, involve recordkeeping and allowing investigators to prepare just the right quantities of buffers or reagent solutions.
A typical system, exemplified by Mettler’s Quantos instrument, employs both solid and liquid dispensing to create solutions of exact concentration in any container. After the system dispenses the solid, it adds precisely enough liquid for the desired concentration. The two-step process eliminates the burden on the technician of weighing solids and dispensing liquids precisely. When the operation is complete, Quantos prints a label describing exactly what is in the vial and records that information.
“The benefits are that you can now make up much smaller volumes of sample,” Dr. Ratcliff tells Lab Manager Magazine. “That’s because the minimum weight is lower than what might be accurately measured by hand, and you don’t need to round up the liquid levels in a volumetric flask.” For example, a worker expecting to make 50 HPLC injections of 10 microliters each can accurately produce one mL of solution instead of having to fill a 10 mL or 100 mL volumetric flask. This approach also shields workers from potentially toxic solids and replaces expensive volumetric glassware, which requires cleaning, with disposable, low-cost vials.
DOLLARS AND CENTS
The cost of automation is always a consideration in lab managers’ decisions on whether to automate. “Some decision makers think they will save money by not automating,” says Mehul Vora, global product manager in Beckman Coulter Life Sciences’ (Brea, CA) automation group. “But in the long run, as automated sample prep becomes a routine part of your lab, the quality improvements and wiser deployment of human resources will pay for themselves in a very short time.”
Real life experience confirms this view. Analysis by Agilent indicates a rapid return on investment for sample and standard prep automation based on the cost of labor, glassware, lower consumption of reagents and solvents, and reduction of rework.
A technician spending just five minutes on each of 300 samples per week costs the lab about $7,000 per month, assuming salary and overhead at $70 per hour. Creating standards “on demand” in appropriate volumes can cut solvent use by 95 percent and reference standard consumption by up to 75 percent. An Agilent customer reported solvent-related savings of $16,000 per year for just one assay, including much lower disposal costs and savings on glassware replacement and cleaning of more than $60,000 per year.
A case study from Horizon Technology summarized in Table 1 illustrates direct cost savings plus labor savings from switching from manual to automated prep.
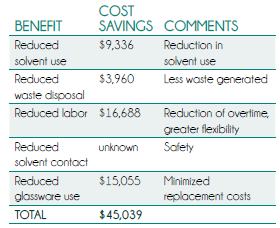 The $45,039 in savings represents an approximately three-year payback on a $141,000 system consisting of an eight-position extraction system, a DryVap add-on for drying and concentrating samples, and vacuum pumps. Direct ROI was only a minor part of the equation for this customer, however. They estimated that the automation capability enabled them to take on approximately $150,000 per year in more-complex contract work in addition to retaining Method 525.2 in-house, valued at $117,000 yearly. EPA Method 525.2 is a complex drinking water analysis protocol involving a very large list of analytes. Solid phase extraction enabled this lab to meet the method’s strict quality requirements more easily.
The $45,039 in savings represents an approximately three-year payback on a $141,000 system consisting of an eight-position extraction system, a DryVap add-on for drying and concentrating samples, and vacuum pumps. Direct ROI was only a minor part of the equation for this customer, however. They estimated that the automation capability enabled them to take on approximately $150,000 per year in more-complex contract work in addition to retaining Method 525.2 in-house, valued at $117,000 yearly. EPA Method 525.2 is a complex drinking water analysis protocol involving a very large list of analytes. Solid phase extraction enabled this lab to meet the method’s strict quality requirements more easily.
All the arguments for automating sample preparation are equally valid for standards prep. Standards preparation rivals sample preparation in repetitiveness, reliance on precise measurement, and in many cases, the number of units. While standards prep is somewhat more predictable in terms of operations (dispensing, diluting, etc.), it is no less critical to data quality. Some protocols, particularly in food safety testing, call for preparing dozens of potential reference analytes at several concentrations each.
Automating standards preparation saves reagents and samples by producing the right quantity of standard solutions. As with sample prep, technicians tend to “overdo” standards prep, sometimes creating 10 or 20 times as much stock solution as may be needed. Automated systems can be set up to make up just enough solution for one day’s assays fresh at the beginning of the first shift. “Labs are much less likely to use expired standards when they have an automated solution to do all the work,” observes Agilent’s Peter Mrozinski.
 Deep Well Washer / ELx405 Select / BioTek / www.biotek.com As with sample prep, automating benefits standards prep not so much through speed as by introducing consistency and efficiently utilizing human resources. Raw sample numbers play a surprisingly insignificant role in the decision to automate.
Deep Well Washer / ELx405 Select / BioTek / www.biotek.com As with sample prep, automating benefits standards prep not so much through speed as by introducing consistency and efficiently utilizing human resources. Raw sample numbers play a surprisingly insignificant role in the decision to automate.
“Automation’s contributions are its consistency and reproducibility,” Mrozinski adds. “It’s as accurate as your best technician on a good day but with the reliability and reproducibility one expects from an analytical instrument.”
In November 2012, Agilent introduced the Encore Multispan Liquid Handling System for advanced automated sample preparation. Encore combines innovative pipetting with a built-in robotic arm that automates a substantially larger portion of sample prep workflows while increasing walkaway time.
Equally important for a lab’s business and worker satisfaction, automation creates an atmosphere of accomplishment. “In the end, a robot isn’t necessarily any faster than a skilled technician,” says Jason Greene, product manager at BioTek Instruments (Winooski, VT). “But it does provide uniformity and performance, accuracy, and precision, and it gives workers the opportunity to be elsewhere.”
