Biologists Apply Optogenetics to Cancer for the First Time
Researchers prevent, normalize tumors using light to control cell electric signals
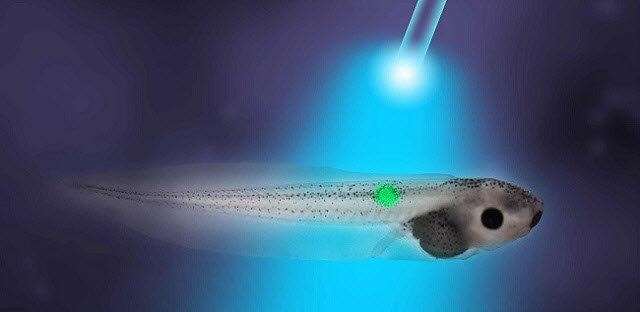 Image courtesy of Tufts University
Image courtesy of Tufts University
MEDFORD/SOMERVILLE, Mass. -- Tufts University biologists using a frog model have demonstrated for the first time that it is possible to prevent tumors from forming and normalize tumors after they have formed by using light to control electrical signaling among cells. The work, which appears online in Oncotarget on March 16, is the first reported use of optogenetics to specifically manipulate bioelectrical signals to both prevent and cause regression of tumors induced by oncogenes.
Frogs are a good model for basic science research into cancer because tumors in frogs and mammals share many of the same characteristics. These include rapid cell division, tissue disorganization, increased vascular growth, invasiveness and cells that have an abnormally positive internal electric voltage.
Virtually all healthy cells maintain a more negative voltage in the cell interior compared with the cell exterior; the opening and closing of ion channels in the cell membrane can cause the voltage to become more positive (depolarizing the cell) or more negative (polarizing the cell). Tumors can be detected by their abnormal bioelectrical signature before they are otherwise apparent.
Related Article: Nanoparticles Target and Kill Cancer Stem Cells that Drive Tumor Growth
"These electrical properties are not merely byproducts of oncogenic processes. They actively regulate the deviations of cells from their normal anatomical roles towards tumor growth and metastatic spread," said senior and corresponding author Michael Levin, Ph.D., who holds the Vannevar Bush chair in biology and directs the Center for Regenerative and Developmental Biology at Tufts School of Arts and Sciences. "Discovering new ways to specifically control this bioelectrical signaling could be an important path towards new biomedical approaches to cancer."
Lead author Brook Chernet, PhD, former post-doctoral associate in the Levin laboratory, injected cells in Xenopus laevis embryos with RNA encoding a mutant RAS oncogene known to cause cancer-like growths. The researchers also expressed and activated either a blue light-activated, positively charged ion channel, ChR2D156A, or a green light-activated proton pump, Archaerhodopsin (Arch), both of which hyperpolarize frog embryonic cells, thereby inducing an electric current that caused the cells to go from a cancer-like depolarized state to a normal, more negative polarized state. Activation of both agents significantly lowered the incidence of tumor formation and also increased the frequency with which tumors regressed into normal tissue.
Related Article: Using Light for Targeted Drug Delivery Could Help Fight Tumors, Local Infections
The use of light to control ion channels has been a ground-breaking tool in research on the nervous system and brain, but optogenetics had not yet been applied to cancer.
"This provides proof of principle for a novel class of therapies which use light to override the action of oncogenic mutations," said Levin. "Using light to specifically target tumors would avoid subjecting the whole body to toxic chemotherapy or similar reagents."
Other authors on the paper were Dany S. Adams, PhD, research associate professor in the Department of Biology, and Maria Lobikin, PhD, recent post-doctoral researcher in the Levin laboratory.
This work was supported by the G. Harold and Leila Y. Mathers Charitable Foundation.
Brook T. Chernet, Dany S. Adams, Maria Lobikin, and Michael Levin, "Use of genetically encoded, light-gated ion translocators to control tumorigenesis," Oncotarget, online March 16, 2016, DOI: 10.18632/oncotarget.8036.
