Several years ago, Natalie had an experience that gave her a new perspective on health and well-being. Having recently been diagnosed with a rare form of Leukemia, Natalie’s best friend was at a loss as to how to face treatment options. As a scientist working in drug development, Natalie was determined to act swiftly to find a solution to her friend’s dilemma. Leveraging her knowledge and experience in oncology, Natalie rigorously researched the disease to better understand the pathogenesis and to evaluate potential therapeutic strategies. At the time, standard treatments for the disease led to remission in only a small percentage of cases. New techniques including hematopoietic stem cell transplantation, while promising, had thus far resulted in outlooks that were less than ideal.
In a eureka moment, Natalie uncovered a promising new development that gave them both hope. With Natalie’s support, her friend started a treatment regimen with a brand new therapeutic that had recently gained FDA approval.1 This novel biologic drug is a first-in-class bispecific antibody that targets both CD3+ T cells and CD19+ tumor cells, helping to mediate upregulation, production, and release of key adhesion and cytokine molecules. Coordinated activities were shown to result in tumor cell lysis, ultimately leading to a potentially positive immunological outcome.
Natalie’s friend was faced with limited choices and a leap of faith that this new drug would be effective. Although seemingly risky at the time, her actions proved successful, and her disease eventually retreated into remission. Feeling fortunate to be able to help her friend, Natalie paused to truly appreciate the value of her work and the impact of the many talented researchers beside her. Importantly, her experience and those of many others at the time highlighted the dawning of a new chapter in immunotherapeutic drug development.
A new era for therapeutic antibodies
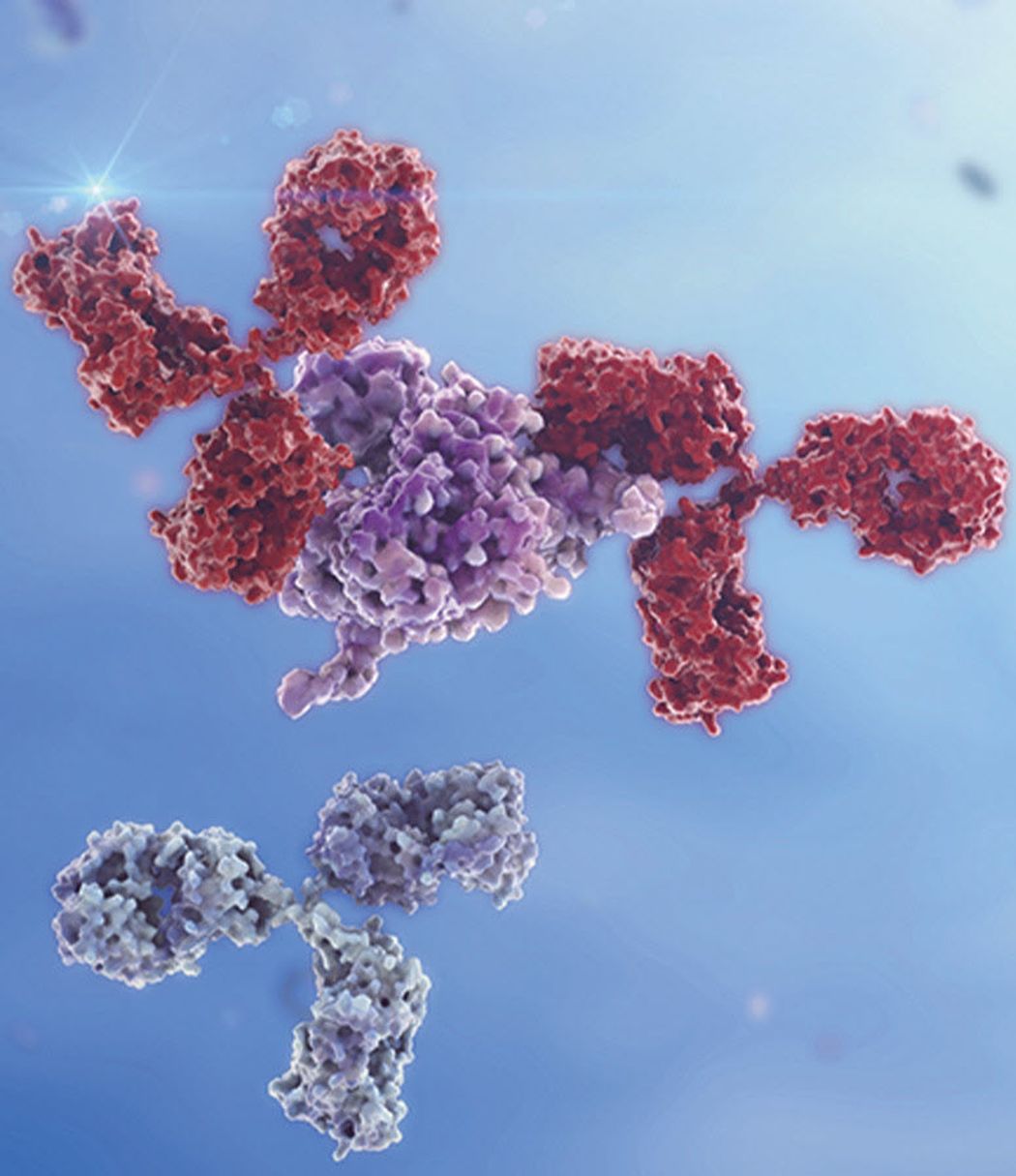
The world of therapeutic antibodies is rapidly evolving, with state-of-the-art research and technologies defining an entirely new landscape. Beyond traditional monoclonal antibodies (mAbs),2 advancements in bi-specific antibodies (BsAbs), tri-specific antibodies (TsAbs), antibody-drug conjugates (ADCs), and other fragment-based antibody derivatives are changing the way diseases like cancer and autoimmune disorders are treated.3
These novel approaches require updated cell line development (CLD) workflows underlying the antibody production process. This has emphasized the need for collaborative solutions, with integrated instruments, software, and services to accelerate CLD workflows and drive faster biotherapeutic development.
The challenges of CLD
iStock, markusblanke
Essential to the process of therapeutic antibody production, CLD is no simple task. Establishing cell lines is a long process with numerous steps, each presenting challenges. As therapeutic approaches continue to evolve, these challenges become more complex—requiring solutions to eliminate bottlenecks and streamline workflows at each step of the CLD process.
“Previously, developing and validating protocols for selecting a high-yield cell line with the desired critical quality attributes (CQAs) for mAB production took one and a half to two years. Today by leveraging platform technologies, biopharma companies have reduced this timeline to six to eight months!” explains Miranda Kheradmand, vice president, strategic marketing & workflow solutions for Life Sciences at Danaher Corporation.
“However, the shift toward bi-specific and tri-specific antibodies—designed to target multiple antigens, potentially extending treatment efficacy, especially in oncology, where tumor adaptation drives resistance—introduces new challenges,” explains Kheradmand. “The goal of these next-generation antibodies is to enhance specificity and potency. However, their structural complexity means that existing platform technologies may not achieve the same yields and/or CQAs.”
New complexities
BsAbs and TsAbs require multiple CLD workflows that run in parallel. Once positive clones are identified for a specific binding activity, the gene vectors are fused through recombinant techniques. The resultant BsAb or TsAb recombinant vectors must then be screened, characterized, and validated to arrive at a high-producing, stable, and robust cell line suitable for cell banking.

iStock, Moyo Studio
Labs invest considerable time and resources in developing cell lines and master cell banks and in establishing platform technologies for mAbs. However, they typically lack historical learning and validated process information for developing newer products like BsAbs and TsAbs. CLD processes for these antibodies are more challenging from logistics and data management perspectives. To accomplish this type of development, labs must do considerably more clone screening, leverage more resources, and complete twice the amount of CLD work.
“Other key factors for CLD are stability and scalability. Biopharmaceutical companies continue to adopt advanced manufacturing technologies, such as bioreactors, to enhance product quality and/or sustainability,” explains Kheradmand. “These changes place extra importance on the need for a robust CLD process.”
“One critical objective for our customers is to reduce drug development timelines. They seek automation, advanced clone screening, analytical technologies, and digital solutions to enable ‘fast failure’—eliminating suboptimal clones early without discarding promising candidates,” states Kheradmand. “To meet these needs, they turn to companies like Danaher for innovative and end-to-end solutions.”
Solutions through collaboration
In the biopharmaceutical setting, therapeutic antibody development and scale up requires close collaboration between three departments. The three groups—analytical core lab, upstream process development, and downstream development—are part of chemistry, manufacturing, and controls (CMC), which is critical to the process of technology transfer to manufacturing.
New technologies can help eliminate bottlenecks, speed analytical testing, and accelerate the CLD process. The ability to leverage these technologies to scale up as the drug moves downstream and eventually into GMP manufacturing is an advantage. Such technologies can make standardization easier, accelerating the tech transfer to manufacturing process.
“When a lab or a company is really thinking about bringing a new technology into their CLD workflow, there are many factors to consider,” explains Kheradmand. “Can this technology provide me with flexibility and scalability needed soon? Can this technology fit seamlessly in the analytical core lab while giving me early access to CQA data? Can it be used in upstream or downstream workflows to enable standardization?”
Having an industry partner who looks at a problem or pain point from the customer’s perspective can be extremely valuable, especially when it comes to challenging CLD workflows. Working with a company that has a diverse, yet integrated portfolio of technology solutions can provide a seamless development process.
“This is the broader vision our operating companies focus on—driving innovation by identifying technologies that address customers’ pain points while taking a holistic, end-to-end approach. By integrating solutions across the entire process, we deliver measurable value aligned with our customers’ KPIs,” explains Kheradmand.

iStock, Moyo Studio
Rather than connecting with many individual companies that each provide a specific solution for a given step in the development process, the right partner can provide a central point of contact for customers to access solutions across the workflows. They can offer a bird’s-eye-view, providing the time and knowledge to evaluate these connections.
“One of the areas that our customers are challenged in is data management, particularly in CLD,” explains Kheradmand. “In CLD a vast amount of data gets generated across multiple labs and platforms. From clone screening to stability assessments, scalability, CQAs, to process optimization, each dataset must be accurately recorded, analyzed, tracked, and stored, with secure access and full traceability—who generated the data, when, how it was transferred, and who has access to it.”
“The complexity grows as data comes from diverse automation platforms, analytical technologies, and global teams that at times are in different locations,” explains Kheradmand. “All of these data must be seamlessly integrated while maintaining regulatory compliance. The key questions we focus on answering are: How can we reduce turnaround time, ease data access, enhance data quality, and ensure compliance across every stage of CLD? Our solutions address these challenges by enabling streamlined, compliant, and high-integrity data management throughout the process.”
Looking to the future
As the world of immunotherapeutics expands to encompass novel antibodies, proteins, and cell and gene therapy approaches, collaborations become even more important in providing the right tools, platform technologies, and expertise.
“We are embedded with our customers. We grow with them, and we focus on innovative solutions that are impactful for our customers and have longevity. I think that’s the critical benefit that Danaher provides,” states Kheradmand. “Identifying the right investment that can be leveraged as the company strategy and direction evolves, for instance, from antibodies to recombinant proteins, to cell and gene therapy.”
Summary
In the end, the objective of immunotherapeutic development is to leverage all resources and expertise to provide better options for the treatment of challenging diseases like cancer. This is the unwavering driving force behind new therapeutic strategies. The right partner can help overcome development challenges and get to the destination faster and more efficiently.
To learn more, visit lifesciences.danaher.com
References
1. “FDA Approval: Blinatumomab.” https://aacrjournals.org/clincancerres/article/21/18/4035/118026/FDA-Approval-BlinatumomabFDA-Approval-of
2. “Monoclonal antibody therapeutics: history and future.” https://pubmed.ncbi.nlm.nih.gov/22920732/
3. “Emerging new therapeutic antibody derivatives for cancer treatment.” https://www.nature.com/articles/s41392-021-00868-x



