In the world of laboratories and pharmaceutical facilities, accurate and precise temperature control isn’t just a regulatory to-do list item—it’s the foundation for reliable science, product integrity, and operational success. Accuracy ensures that samples are kept to the most suitable setpoint, and precision allows even the smallest deviations to be identified and corrected, preventing potential damage. Think about it: every scientist or lab manager has experienced the sinking feeling of a compromised sample or an unexplained anomaly in their results. Often, the root cause points back to one factor: temperature inconsistency.
This is where temperature mapping takes center stage. It’s not just a process; it’s your guarantee that every shelf, compartment, and corner of your controlled temperature unit, or CTU, is doing exactly what it’s supposed to do—maintaining consistent conditions.
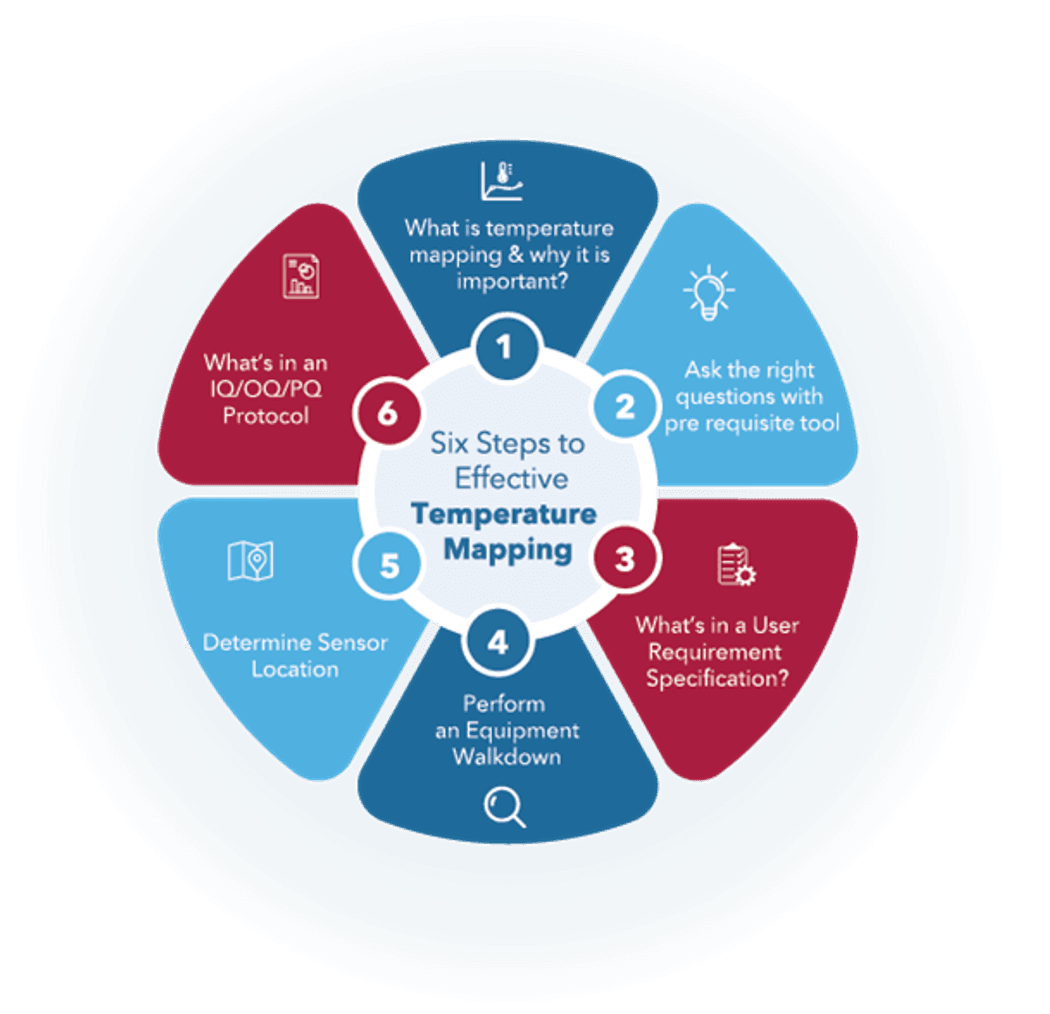
More than a regulatory requirement, it’s an investment in confidence, preserving sample integrity, and safeguarding your lab’s reputation. This article explores the essentials of effective temperature mapping with a six-step process.
Laying the foundation: Six steps to effective temperature mapping
Temperature mapping is the foundation for maintaining quality and compliance in environments where temperature-sensitive products are stored. This process offers a structured approach to ensure your mapping efforts are thorough, effective, and aligned with regulatory requirements. By following these steps, lab and pharma managers can mitigate risks, improve operational efficiency, and safeguard the integrity of their products.
Credit: Nathan Roman
Understanding temperature mapping: What it is and why it matters
Temperature mapping evaluates temperature consistency in controlled environments—such as freezers, incubators, and warehouses—by strategically placing calibrated sensors. Ensuring precision and accuracy in sensor placement and calibration is essential for detecting hotspots, cold spots, and fluctuations that can compromise temperature-sensitive materials, including pharmaceuticals, biological samples, and reagents. For example, door openings in an ultra-low-temperature freezer may create localized variations that affect vaccine efficacy if not precisely identified and accurately measured.
For lab managers, ensuring both precision and accuracy in temperature mapping is crucial. Even brief deviations outside acceptable ranges can degrade materials, alter potency, and shorten shelf life. Reliable, high-quality sensors and careful methodology confirm stable conditions, meeting FDA, EMA, and WHO requirements while safeguarding patient safety and maintaining the organization’s reputation.
Beyond compliance, precise and accurate temperature mapping data can help optimize storage systems and improve energy efficiency, delivering cost savings and operational excellence. In short, temperature mapping—executed with exacting standards—supports product integrity, organizational credibility, and regulatory peace of mind.
Asking the right questions: Determining prerequisites for drafting protocols
With a clear understanding of why temperature mapping matters, the next step is preparation. Before drafting protocols, it's essential to define the scope and purpose of the study. Are you validating a new freezer, assessing a warehouse's suitability for biologics, or remapping an incubator after repairs? Identifying the equipment owner or primary user and understanding its process range, operating setpoints, and alarm setpoints are crucial steps in ensuring a successful mapping project.
Precision in defining these prerequisites is essential to ensure the mapping process captures accurate and meaningful data. For instance, specifying precise measurement intervals and acceptable temperature tolerances ensures that even minor variations are detected, allowing lab managers to address potential issues proactively. Without such precision, mapping results may overlook subtle fluctuations that could compromise products or regulatory compliance.
It’s also important to determine the regulatory framework and any specific operational constraints. For instance, FDA regulations might demand tighter control in a freezer storing vaccines, while EMA guidelines could recommend specific mapping frequencies to maintain compliance for freezers or refrigerators. Then, understanding the equipment’s dimensions, layout, and existing monitoring systems ensures that protocols align with both compliance requirements and operational realities.
Taking time to ask these foundational questions minimizes errors, helps anticipate challenges, and ensures a streamlined mapping process that delivers actionable insights. As you move forward, keep these considerations documented to provide a clear reference for stakeholders throughout the project.
User requirement specification (URS): Establishing performance criteria
Building on the groundwork established in the preparation phase, the user requirement specification (URS) serves as the blueprint for your mapping project. It formalizes the objectives and expectations, ensuring alignment across teams and stakeholders. For example, a URS might specify that a refrigerator’s temperature must remain between 2°C and 8°C, with no variation greater than ±0.5°C during normal operation.
A robust URS should outline critical parameters such as sample capacity, predefined setpoints, temperature operating range, alarm limit capabilities, and acceptable performance criteria. It should also detail the equipment and software required, ensuring compatibility and accuracy.
The URS acts as a guiding document, ensuring that mapping efforts are focused, efficient, and compliant with regulatory expectations. Sharing this document with third-party validation providers or internal teams promotes transparency and reduces the risk of miscommunication.
Equipment walkdown: Documenting physical and functional aspects
Once the URS is in place, the next step is to conduct an equipment walkdown. This process involves a detailed inspection of the unit to identify potential risks and gather critical data for planning.
Key tasks during the walkdown include evaluating air circulation patterns, assessing the condition of existing monitoring systems, and verifying maintenance records. Addressing any deficiencies—such as worn gaskets on freezer doors or misaligned vents—ensures that the environment is in optimal condition before mapping begins.
A comprehensive walkdown not only enhances the accuracy of your study but also reduces the likelihood of encountering issues during data collection. Be sure to document your findings, as they will directly inform the placement of sensors and other critical steps in the process.
Sensor placement: Determining quantity and location
With the walkdown complete, the focus shifts to strategic sensor placement. The effectiveness of temperature mapping depends on capturing an accurate thermal profile, and this begins with identifying key areas for monitoring. Sensors should be placed in locations representing both typical conditions and potential problem zones. Precise sensor placement, such as positioning at airflow vents or near door seals, ensures accurate monitoring of potential problem zones. A lack of precision in sensor placement can result in blind spots, where localized deviations remain undetected, jeopardizing product stability. Regulatory guidelines suggest aligning sensor placement with areas prone to variability, like corners and high-use zones, to capture a complete thermal profile. For ultra-low freezers, this could mean placing sensors adjacent to the control probe or where product loads create thermal barriers.
Regulatory guidelines, or industry good practice guides, can often help to determine the factors which impact the number and placement of sensors in a unit. Calibration is equally critical; using uncalibrated sensors can compromise data accuracy and undermine the validity of the study.
Proper sensor placement not only provides insights into temperature uniformity but also ensures that no critical areas are overlooked. The resulting data empowers you to make informed decisions about the suitability of the unit for its intended purpose.
Qualification protocols (IQ, OQ, PQ): Ensuring performance through robust protocols
The final step in the process is executing qualification protocols: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These protocols validate that the equipment performs reliably and consistently within defined parameters.
The IQ confirms that the unit has been installed correctly and that all components, such as sensors, controllers, and alarms, meet design specifications. The OQ evaluates the unit under standard operating conditions, often testing for resilience by simulating scenarios like door openings or power outages.
High-precision sensors play a critical role in these processes, enabling detailed temperature mapping data analysis that helps lab managers pinpoint specific areas of concern. For example, a localized hotspot detected during mapping could prompt adjustments in airflow patterns, ensuring uniform cooling and preventing product degradation.
Finally, the PQ validates performance over an extended period under real-world conditions, ensuring the equipment consistently meets operational requirements. Ensuring the precision of data collected in these qualifications not only satisfies regulatory requirements but also provides actionable insights for long-term operational improvements.
By completing these qualifications, you provide documented evidence of the unit’s suitability for its intended use. This step ensures regulatory compliance, protects product quality, and delivers confidence in the reliability of your temperature-controlled environment.
Take control of your temperature mapping today
By following these six steps—beginning with a solid understanding of temperature mapping and ending with robust qualification protocols—you can ensure the success of your temperature mapping. In other words, laying the foundation for effective temperature mapping.
Now is the perfect time to take a closer look at your temperature mapping practices. Are you confident that your current protocols meet regulatory standards and protect your products? Have you recently reviewed your equipment, sensor placement, or qualification processes to identify potential gaps or areas for improvement?
If you’re unsure, don’t wait until an audit or product failure highlights the need for action. Take charge of your temperature mapping today—your products, patients, and processes depend on it.













