Authenticity of fruit juices and purees by LC-MS/MS
Authors: Julie Moriceau1, Jean-Francois Garnier1, Scott Peterman2, Neloni Wijeratne2, Claudia Martins2, Sylvain Morel1
1Thermo Fisher Scientific Customer Solution Center, Villebon/Yvette (91), France
2Thermo Fisher Scientific, San Jose, California
Keywords: Polymethoxylated flavones, limonin, phenols, fruit juice, authenticity, LC-MS/MS method, TSQ Fortis Plus mass spectrometer, Hypersil GOLD VANQUISH column
Goal
To develop an analytical method that merges three conventional LC-UV methods into one LC-MS/MS method that incorporates polarity switching, improving laboratory productivity while maintaining high data confidence.
Introduction
According to the European Association of the Industry of Juices and Nectars (AIJN), 9.1 billion liters of fruit juices and nectars were consumed across the EU in 2018. Fruit juices and concentrates are natural products with a very limited range of allowed additives. Unfortunately, some juice products or fruit purees may be adulterated easily either by dilution with water and other juices or by adding specific components, such as acids and sugars.1
Diversity in adulteration techniques, natural variation due to geographical origins and species, as well as storage conditions and processing techniques make the detection and prevention of juice adulteration an analytical challenge. In that scope, this study describes a single LC-MS/MS approach for monitoring specific nutraceutical markers like polymethoxylated flavones (PMF), limonin, and phenols
in various juices and fruit purees. The objective is to simultaneously measure the following compounds: arbutin (phenols), phloridzin (phenols), 7-methoxycoumarin (PMF), limettin (PMF), bergapten (PMF), isopimpinellin (PMF), sinensetin (PMF), tangeritin (PMF), nobiletin (PMF), and limonin.
Currently, three separate methods are used to analyze all of the listed nutraceuticals. Each method is used to analyze subsets of compounds due to ionization potential (cation or anion formation) and the chromatographic conditions. In some instances, different columns and mobile phases may be needed to differentially retain all target compounds.
Experimental
Sample preparation
The calibration range was different for each compound. As some molecules are very hydrophobic (practically insoluble in water), the dilutions were made in a 50/50 (V/V) mix of methanol and water. Nine standards were made by serial dilutions as shown in Table 1.
These markers are the fingerprints of the juice. The presence and concentration of these compounds in juice depend on the nature of fruit as well as the geographical and varietal origin. Different sample preparations were made as a function of the type of matrices as not all the compounds were present in every matrix (Table 2).
PMF | Limonin | Arbutin | Phloridzin |
Standard 1 | 1.25 | – | – | – |
Standard 2 | 2.5 | – | – | – |
Standard 3 | 5 | – | – | – |
Standard 4 | 12.5 | – | – | – |
Standard 5 | 25 | 20 | 125 | 62.5 |
Standard 6 | 50 | 40 | 250 | 125 |
Standard 7 | 125 | 100 | 625 | 312.5 |
Standard 8 | 250 | 200 | 1250 | 625 |
Standard 9 | 500 | 400 | 2500 | 1250 |
Table 1. Standards concentrations in ng/mL
Table 2. Research compounds in each of the matrices
Type of matrices | Compounds to quantify |
Orange juice | PMF and limonin |
Lime juice | PMF and limonin |
Apple puree | Phenols |
Pear puree | Phenols |
For the PMF and limonin analysis, the sample was homogenized then an aliquot of 2 mL was transferred and centrifuged at 4,500 rpm for 5 minutes. The supernatant was diluted ten-fold (for samples for PMF analysis) and fifty-fold (for samples for limonin analysis) with a 50/50 (V/V) mix of methanol/water.
For the phenols analysis, the sample was homogenized and diluted ten-fold with a 50/50 (V/V) mix of methanol/ water. It was then filtered with a 0.2 µm syringe filter.
LC conditions
LC analysis was performed on a Thermo Scientific™ Vanquish™ Flex Binary UHPLC system. The column used was a Thermo Scientific™ Hypersil GOLD™ VANQUISH™ column (100 × 2.1 mm, 1.9 µm particle size) maintained at 30 °C. Mobile phases A and B consisted of 2 mM ammonium formate with 0.05% formic acid in Fisher Chemical™ Optima™ grade water and methanol, respectively. The total UHPLC gradient was 13 minutes (Table 3), with 0.5 µL injection volume.
Table 3. UHPLC gradient information
Time (min) | Flow rate (mL/min) | B (%) |
0.00 | 0.400 | 5.0 |
3.00 | 0.400 | 80.0 |
6.00 | 0.400 | 100.0 |
8.00 | 0.400 | 100.0 |
8.10 | 0.400 | 5.0 |
13.00 | 0.400 | 5.0 |
MS conditions
The Thermo Scientific™ TSQ Fortis™ Plus triple-stage quadrupole mass spectrometer was used for this analysis. All compounds for this study were analyzed in positive and negative heated electrospray mode (HESI). The experimental conditions were optimized with a static spray voltage, and a cycle time of 0.5 s, while Q1 and Q3 resolutions were maintained at 0.7 and 1.2 Da FWHM, respectively. The source parameters and SRM table along with other critical MS features for all the target analytes are listed in Tables 4 and 5, respectively. Individual standards were infused into the mass spectrometer to determine the optimum tube lens settings and collision energies and source fragmentation voltage for the product ions. Timed-SRM scan mode and automated dwell time calculation (based on the expected peak width and desired number of data points across the peak) help users to easily set up instrument parameters and at the same time get maximum performance (sensitivity and chromatographic resolution).
Software
Data acquisition and processing were conducted using Thermo Scientific™ TraceFinderTM software version 5.1. Additionally, Thermo Scientific™ Chromeleon™ 7.3 Chromatography Data System (CDS) can be used for data processing and provides efficient and compliant software to manage all analytical processes, from instrument control to final report generation.
Table 4. Source parameters for analysis of nutraceuticals on the TSQ Fortis Plus triple quadrupole mass spectrometer
Ion source parameter | Value |
Spray voltage | Static |
Positive ion | 1,500 V |
Negative ion | 2,000 V |
Sheath gas | 50 Arb |
Aux gas | 10 Arb |
Sweep gas | 1 Arb |
Ion transfer tube temperature | 300 °C |
Vaporizer temperature | 350 °C |
CID gas | 1.5 mTorr |
Table 5. Optimized mass spectrometer transitions for the nutraceuticals with retention time of 6.5 min and retention time window of 13 min
Compound | Polarity | Precursor (m/z) | Product (m/z) | Collision energy (V) | Tube lens (V) | Source fragmentation (V) |
Arbutin | Negative | 317.02 | 108.16 | 25 | 52 | 5 |
Arbutin | Negative | 317.02 | 271.14 | 5 | 52 | 5 |
Phloridzin | Negative | 435.128 | 167.11 | 25 | 71 | 19.6 |
Phloridzin | Negative | 435.128 | 273.16 | 15 | 71 | 19.6 |
7?methoxycoumarin | Positive | 177.05 | 121.08 | 20 | 62 | 21.2 |
7?methoxycoumarin | Positive | 177.05 | 133.18 | 15 | 62 | 21.2 |
Limonin | Positive | 471.201 | 161.02 | 35 | 94 | 13.1 |
Limonin | Positive | 471.201 | 425.15 | 20 | 94 | 13.1 |
Ispimpinellin | Positive | 247.06 | 216.99 | 25 | 72 | 14.7 |
Ispimpinellin | Positive | 247.06 | 231.96 | 20 | 72 | 14.7 |
Limettin | Positive | 207.065 | 164.05 | 25 | 73 | 18 |
Limettin | Positive | 207.065 | 192.04 | 20 | 73 | 18 |
Bergapten | Positive | 217.049 | 174.03 | 30 | 76 | 18 |
Bergapten | Positive | 217.049 | 201.967 | 20 | 76 | 18 |
Sinensetin | Positive | 373.128 | 312.05 | 25 | 80 | 11.4 |
Sinensetin | Positive | 373.128 | 343 | 30 | 80 | 11.4 |
Nobiletin | Positive | 403.138 | 373.05 | 30 | 78 | 9.8 |
Nobiletin | Positive | 403.138 | 388.05 | 20 | 78 | 9.8 |
Tangeritin | Positive | 373.128 | 183.03 | 45 | 81 | 4.9 |
Tangeritin | Positive | 373.128 | 343 | 25 | 81 | 4.9 |
Results and discussion
Due to the presence of isobaric compounds like sinensetin and tangeritin (similar parent and daughter ions) in the mix, a good chromatographic separation is critical for quantitation purposes. Different columns and mobile phase compositions were thoroughly evaluated to obtain the most appropriate separation. The use of a Hypersil GOLD VANQUISH column resulted in robust and consistent separation of sinensetin and tangeritin as shown in Figure 1.
Figure 1. Separation of two isobaric compounds, sinensetin and tangeritin
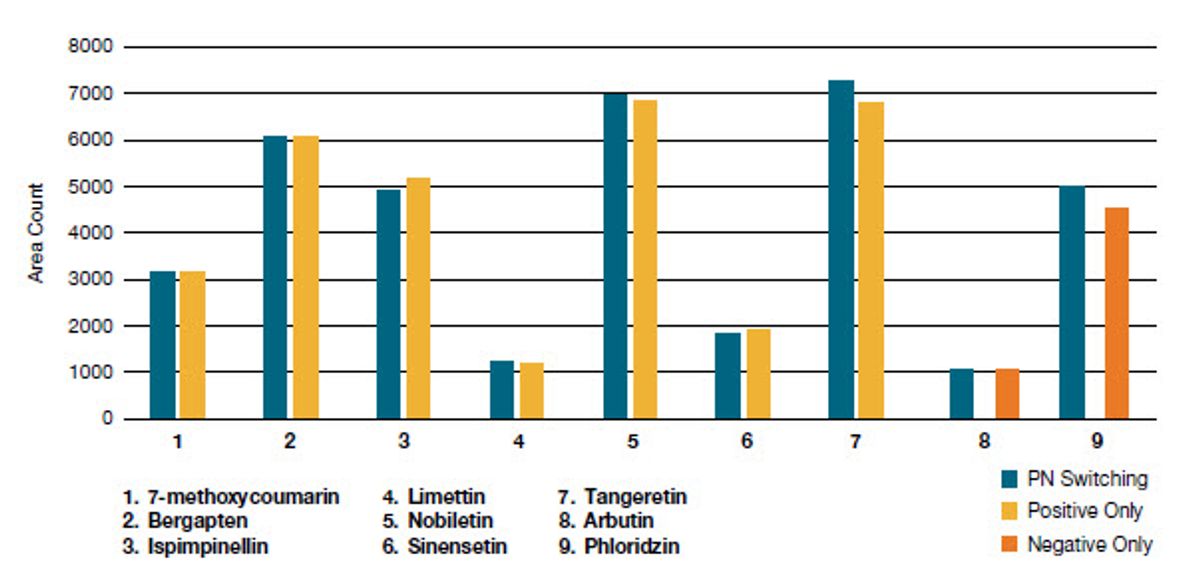
With the increasing demand for greater sample throughput, advancement in triple quadrupole technology is required. One of the ways of increasing sample throughput is by decreasing the time spent on polarity switching. With the new TSQ Fortis Plus MS, a polarity switching speed of 5 ms is possible for better cycle time management. The current method for this study allows the analysis of phenols (negative mode) as well as limonin and PMFs (positive mode) in a single run. Figure 2 shows the detection of all markers in a standard solution (1.25 ng/mL for PMF, 40 ng/mL for limonin, 62.5 ng/mL for phloridzin, and 125 ng/mL for arbutin) using this method. Table 6 and Figure 3 show excellent reproducibility when experiments were carried out both with and without polarity switching at dwell times less than 5 ms. This will allow the user to run methods with both polarities at a shorter LC gradient or addition of more transitions to the method without compromising confidence in quantitation.
Figure 2. Positive and negative extracted ion chromatograms at LLOQ level (a) arbutin, (b) phloridizin, (c) 3-methoxycoumarin, (d) limonin, (e) ispimpinellin, (f) limettin, (g) bergapten, (h) sinensetin, (i) nobiletin, and (j) tangeritin
Figure 2 (continued). Positive and negative extracted ion chromatograms at LLOQ level (a) arbutin, (b) phloridizin, (c) 3-methoxycoumarin, (d) limonin, (e) ispimpinellin, (f) limettin, (g) bergapten, (h) sinensetin, (i) nobiletin, and (j) tangeritin
Table 6. Area count comparison for polarity switching experiment against Cal I and 5 levels for positive and negative mode only experiments
Compound | PN switching | Positive only | Negative only |
Area | %RSD | Area | %RSD | Area | %RSD |
7?methoxycoumarin | 3148.2 | 10.0 | 3151.6 | 8.5 | NA | NA |
Bergapten | 6071.8 | 3.2 | 6063 | 5.9 | NA | NA |
Ispimpinellin | 4917 | 6.5 | 5181.8 | 6.9 | NA | NA |
Limettin | 1215.8 | 8.4 | 1207 | 6.9 | NA | NA |
Nobiletin | 6948.8 | 2.0 | 6858.4 | 1.9 | NA | NA |
Sinensetin | 1828.8 | 8.4 | 1928.4 | 6.2 | NA | NA |
Tangeritin | 7250.2 | 4.8 | 6788.2 | 8.9 | NA | NA |
Arbutin | 1078.4 | 3.1 | NA | NA | 1048.2 | 2.7 |
Phloridzin | 4984 | 3.5 | NA | NA | 4521.4 | 5.2 |
Figure 3. Area count comparison for polarity switching experiment against Cal I and 5 levels for positive and negative mode only experiments, respectively
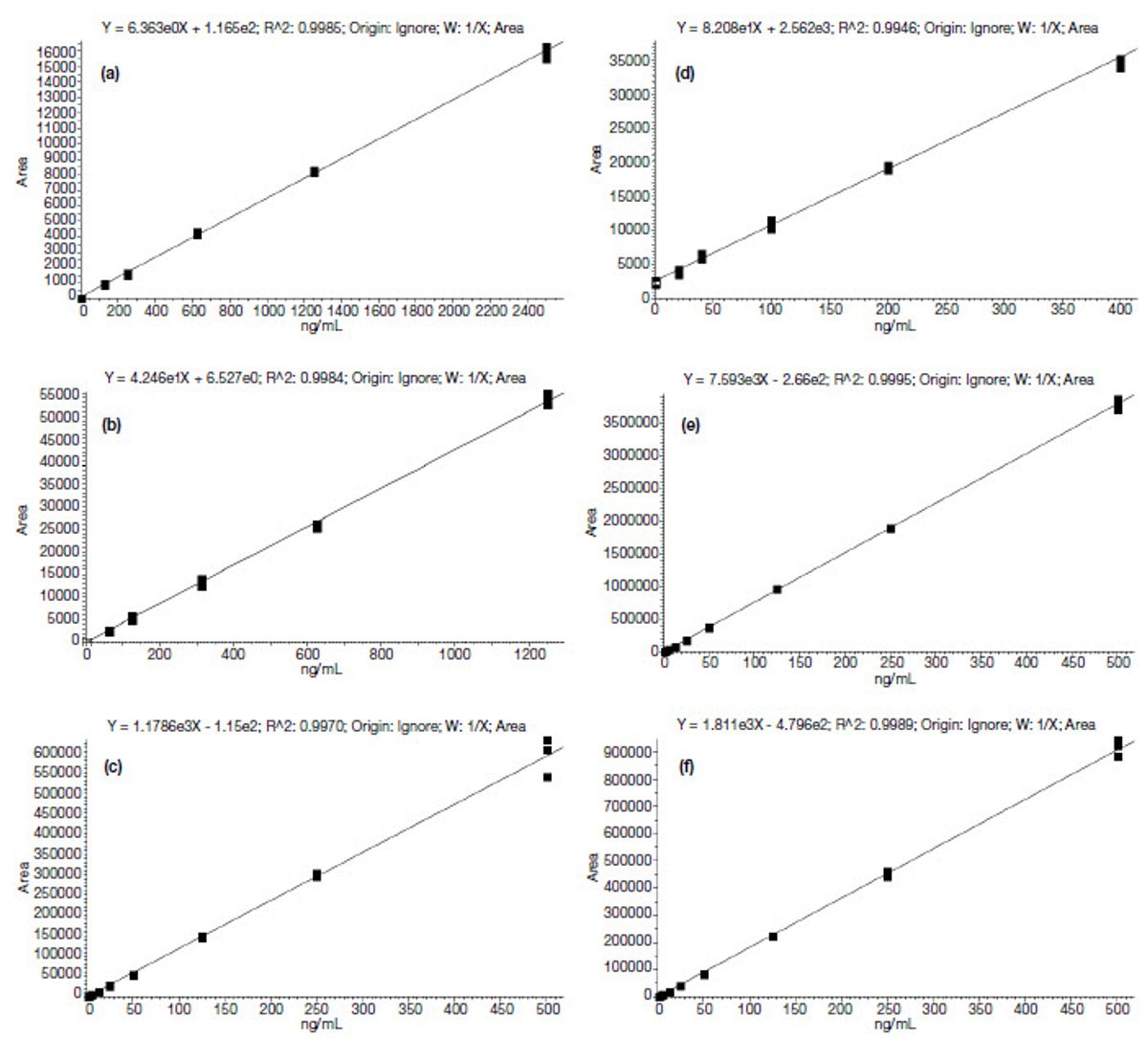
MS calibration curves fit with a linear model for all PMF compounds, limonin, and phenol compounds were used. The calibration curve plotted with the data satisfied the following criteria: R2 value of ≥ 0.98 and with variability (%CV) of < 15%. The percent accuracy of the ion ratio for each sample was averaged at ± 20%, and the required signal-to-noise ratio had a minimum threshold of 3:1.
All calibration details and lower limit of quantification (LLOQ) are displayed in Table 7 and Figure 4.
Arbutin is a glycosylated hydroquinone, which is not very sensitive and most of the time not retained with a reversed phase column. Appropriate chromatographic conditions provide sufficient retention for this compound. The TSQ Fortis Plus MS sensitivity in negative mode helps to detect arbutin at the targeted concentration level without any compromise regarding analysis of other markers. Figure 5 displays a chromatogram of arbutin at 1,250 ng/mL (at least four times less than the amount detected in purees).
Table 7. External calibration curve details
Compound | Polarity | Transition | Calibration range (ng/mL) | R2 | LLOQ (ng/mL) |
Arbutin | Negative | 317 ? 271 | 125 to 2500 | 0.9985 | 125 |
Phloridizin | Negative | 435 ? 273 | 62.5 to 1250 | 0.9984 | 62.5 |
7?methoxycoumarin | Positive | 177 ? 121 | 1.25 to 500 | 0.9970 | 1.25 |
Limettin | Positive | 207 ? 192 | 1.25 to 500 | 0.9989 | 1.25 |
Bergapten | Positive | 217 ? 201 | 1.25 to 500 | 0.9988 | 1.25 |
Isopimpinellin | Positive | 247 ? 216.9 | 1.25 to 500 | 0.9995 | 1.25 |
Sinensetin | Positive | 373 ? 312 | 1.25 to 500 | 0.9972 | 1.25 |
Tangeritin | Positive | 373 ? 343 | 1.25 to 500 | 0.9989 | 1.25 |
Nobiletin | Positive | 403 ? 373 | 1.25 to 500 | 0.9995 | 1.25 |
Limonin | Positive | 471 ? 425 | 40 to 400 | 0.9946 | 40 |
Figure 4. Calibration curves for the 10 compounds (a) arbutin, (b) phloridizin, (c) 3-methoxycoumarin, (d) limonin, (e) ispimpinellin, (f) limettin, (g) bergapten, (h) sinensetin, (i) nobiletin, and (j) tangeritin
Figure 4 (continued). Calibration curves for the 10 compounds (a) arbutin, (b) phloridizin, (c) 3-methoxycoumarin, (d) limonin, (e) ispimpinellin, (f) limettin, (g) bergapten, (h) sinensetin, (i) nobiletin, and (j) tangeritin
Figure 5. Chromatogram of arbutin’s solution standard at a concentration of 1,250 ng/mL
Different sample matrices (orange juice, lime juice, apple puree, and apple pear puree) have been analyzed to confirm the performance of this method and its compliance with conventional method validation criteria. Analytical results have been cross-confirmed by an external reference partner laboratory using a conventional three run approach (data not shown).
Table 8 shows that the pattern of PMF, phenols, and limonin in several juices is very different from one sample to the other and allows accurate differentiation of them. The adulteration by adding water or other cheaper juices would easily be identified.
Table 8. Comparison of amounts obtained for different type of juices and purees (all results are given in ng/mL)
As only a simple dilution is performed for sample preparation, a reproducibility test has been performed. Peak areas of the four nutraceuticals detected in orange juice showed remarkable reproducibility over twenty consecutive injections. Calculated precisions for limonin, sinensetin, tangeritin, and nobiletin were 4.6%, 2.5%, 2.2%, and 2.0%, respectively. Figure 6 shows the response stability, sample after sample.
The combining of three separate methods for PMF, phenols, and limonin into one method using a shorter LC gradient and faster polarity switching speed leads to an impressive productivity improvement.
Compound | Orange juice | Lime juice | Apple puree | Pear puree |
Arbutin | 0 | 0 | 0 | 5714.3 |
Phloridizin | 0 | 0 | 2535.8 | 0 |
7?methoxycoumarin | 0 | 0 | 0 | 0 |
Limettin | 0 | 40.7 | 0 | 0 |
Bergapten | 0 | 27.6 | 0 | 0 |
Isopimpinellin | 0 | 155.6 | 0 | 0 |
Sinensetin | 195.8 | 0 | 0 | 0 |
Tangeritin | 56.8 | 0 | 0 | 0 |
Nobiletin | 271.2 | 0 | 0 | 0 |
Limonin | 444.6 | 906.3 | 0 | 0 |
Figure 6. Twenty consecutive runs of orange juice showing reproducible area counts. %RSD of 4.6%, 2.5%, 2.2%, and 2.0% for limonin, sinensetin, tangeritin and nobiletin, respectively.
Conclusion
This method provides a very robust analytical solution to authenticate fruit juices and purees in only 13 minutes by combining three Thermo Scientific premium products: the Hypersil GOLD VANQUISH 100 × 2.1 mm 1.9 µm LC column, the Vanquish Flex Binary UHPLC system, and the TSQ Fortis Plus mass spectrometer.
It also demonstrates how to drastically increase laboratory productivity in food authentication by merging three conventional LC methods into a single LC-MS/MS method. Several factors have contributed to this:
The simplicity of sample preparation involving a simple dilution
A unique column and a single mobile phase composition for retaining all compounds and appropriately separating the two isobaric ones
The polarity switching efficiency of TSQ Fortis Plus mass spectrometer for monitoring all compounds in positive and negative mode in the same run
The sensitivity of the TSQ Fortis Plus MS system in SRM mode for reaching target LOQs
Easy instrument method setup using timed-SRM and automated dwell time adjustment
Robustness and stability of the overall configuration after multiple injections of juices or purees
Reference
1. FI Handbook on Food Authenticity Issues and Related Analytical Techniques https://secure.fera.defra.gov.uk/foodintegrity/index.cfm?sectionid=83
Find out more at
thermofisher.com
For Research Use Only. Not for use in diagnostic procedures. © 2022 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries. This information is presented as an example of the capabilities of Thermo Fisher Scientific Inc. products. It is not intended to encourage use of these products in any manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change. Not all products are available in all locations. Please consult your local sales representative for details. AN000392-NA-EN 0122S