When it comes to lab safety, many managers default to policies, training, and personal protective equipment (PPE). These are familiar, visible, and often mandated. But they’re also some of the least effective ways to manage risk. Instead, making lab safety decisions can be a much more structured and effective process with the hierarchy of hazard controls.
The hierarchy of hazard controls is a structured approach to implementing different measures of harm prevention.
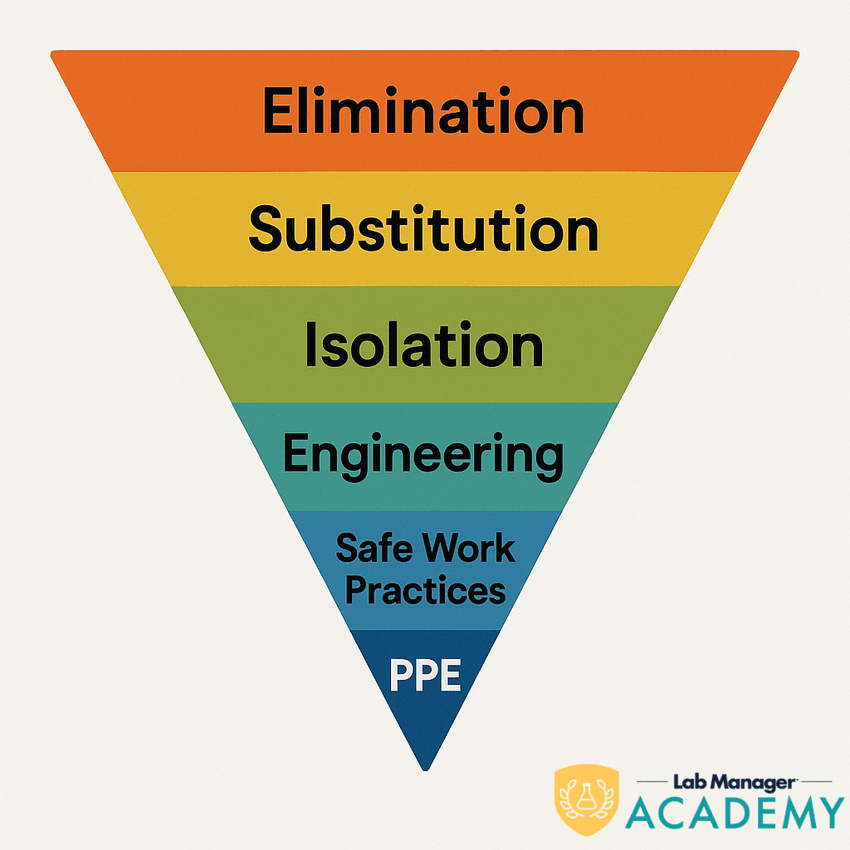
To some, the hierarchy is unintuitive. There are two precepts to understanding it:
- Control the hazard as much as possible, and
- Keep the hazard as far away from the person as possible
So, the hierarchy starts with the control that most effectively fulfills those precepts (eliminating it entirely) and ends with the control that least effectively fulfills them (PPE, which means direct interaction with the hazard).
Ultimately, the hierarchy of hazard controls can help lab managers to stop relying on less effective measures and instead build a safety culture rooted in prevention, not protection alone.
Traversing the hierarchy of hazard controls
Here are overviews of each level of the hierarchy of hazard controls:
Elimination: Should this hazard exist in the lab at all?
Key decision: Can we completely remove this hazard from our lab environment?
Elimination is the most effective form of hazard control because it removes the risk entirely. Yet it’s often overlooked in laboratories, where safety efforts focus on how to manage hazards rather than whether to allow them in the first place.
Lab managers should regularly ask:
- Can this procedure be avoided?
- Are there legacy chemicals or outdated equipment that no longer serve a purpose?
- Do we really need to work with this particularly high-risk material?
Examples include choosing not to purchase highly toxic or unstable chemicals, removing unguarded machinery, or sunsetting research involving high-containment pathogens. These are not just safety decisions; they are operational and strategic decisions, too.
Substitution: Is there a safer alternative?
Key decision: Can we replace this material or method with something less hazardous?
If elimination isn’t feasible, substitution is the next best option. While most applicable to chemical hazards, it can apply to any risk factor, such as biological agents, radioactive materials, and even physical energy sources like noise or temperature.
Effective substitutions reduce risk without compromising research goals. For example:
- Switching from flammable to combustible solvents
- Using lower concentrations of acids
- Choosing less pathogenic organisms for biological studies
Managers must weigh these decisions in partnership with researchers and procurement staff. It often requires reevaluating workflows or sourcing alternatives, but the long-term payoff is fewer downstream controls and fewer points of failure.
Isolation: Can we separate the hazard from the person?
Key decision: Can this hazard be physically separated from personnel?
Though less commonly discussed, isolation plays a vital role in protecting staff. It involves enclosing the hazard source (or the person) to reduce exposure. In lab environments, this could include:
- Using gloveboxes for manipulations involving toxic compounds
- Shielding high-energy equipment
- Housing noisy or radioactive equipment in separate rooms
- Conducting work in BSL3 or BSL4 containment labs
Isolation should be considered during space design or equipment placement decisions. It’s especially valuable for managing persistent or unavoidable hazards that cannot be eliminated or substituted.
Engineering controls: Can we design out the risk?
Key decision: What physical systems can we implement to reduce exposure automatically?
Engineering controls are robust, consistent, and largely independent of human behavior. That makes them especially attractive to lab managers. Once installed, they work, provided they are maintained and used correctly. They are also required by OSHA in many cases, but even if not required, they should still be considered as possible solutions.
Common examples include:
- Fume hoods and biosafety cabinets
- Local exhaust ventilation
- Laser interlocks and shielding
- Ground-fault circuit interrupters (GFCIs)
- Lockout/tagout systems
- Fire suppression systems
These solutions often require upfront investment and infrastructure planning, but they offer significant returns in risk reduction. Managers should consider engineering controls early when building or renovating labs, not as an afterthought.
Work practices: Are we relying too much on behavior?
Key decision: Are staff being trained and consistently expected to work in the safest possible way?
Work practice controls depend on how tasks are performed. They’re often informal, vary by individual, and are prone to drift over time. That makes them less reliable, though still important.
Examples include:
- Proper handwashing protocols
- Keeping fume hood sashes low
- Using tools correctly
- Avoiding direct smell tests (e.g., not wafting chemicals)
Lab managers should continuously evaluate whether these practices are enforced and whether they’re supported by upstream controls. If procedures are the only thing keeping people safe, it may be time to reassess the overall control strategy.
Administrative controls: Is our paperwork driving action?
Key decision: Are our policies and procedures being followed or just filed?
Administrative controls encompass all written documentation—SOPs, safety data sheets, training records, signage, and more. While necessary for compliance and consistency, they suffer from a critical limitation: they don’t control hazards; they control behavior.
This distinction matters. Just because a hazard is listed in a policy doesn’t mean it’s actively being managed. Lab managers must routinely verify that administrative measures translate into daily practice and update them as conditions change.
PPE: Are we using this as a crutch?
Key decision: Are we relying on PPE because we haven’t implemented better controls?
PPE is often the first control applied in labs—and that’s a problem. It should be the last line of defense, used only when upstream controls can’t fully mitigate the hazard.
PPE is prone to failure due to:
- Improper use
- Poor maintenance
- Incorrect selection
- Lack of training
Examples include safety goggles, gloves, lab coats, face shields, and respirators. While essential in many labs, PPE should never be the only barrier between workers and harm.
Lab managers must ask: If PPE is doing all the work, what upstream controls have we skipped?
Stop using the hierarchy upside-down
The hierarchy of controls is more than a diagram; it’s a decision-making tool. Yet many labs invert it, reaching for policies and PPE first instead of asking what can be eliminated, substituted, isolated, or engineered away.
A strong safety culture isn’t built on checklists or lab coats. It’s built on intentional decisions to prioritize prevention over protection, systems over individuals, and design over documentation.
This article was adapted from a Lab Manager Academy Safety Management course on the same subject. Learn more about the Safety Management Certificate at Lab Safety Management Certificate – Ensure a Safer Workplace.














