Humidity can significantly impact the properties of a material, such as its cosmetic surface, and mechanical and chemical properties. Temperature and relative humidity (RH) must be carefully controlled in numerous fields of research, from designing energy storage systems to developing stable pharmaceutical ingredients. Although the two are intrinsically linked, temperature is often given greater consideration in experimental design than humidity, which is more challenging to control with high accuracy and reproducibility.
Strategies for controlling humidity vary, with many researchers using a chamber containing salts (e.g. NaCl, RH 75%) to achieve constant RH. However, these methods are not designed to provide controlled humidity variation and as such, designing experiments with RH as a controlled variable is challenging. Dedicated humidity control systems are increasingly used with analytical methods such as light microscopy, Raman, Fourier transform infrared (FT-IR) spectroscopy, and X-ray to characterize how materials behave in different environmental conditions. Advanced systems are now able to control humidity across 5%–90% RH at a range of temperatures, allowing researchers to precisely control water vapor in the environment surrounding the sample for long periods of time.
Here, we demonstrate the importance of controlling humidity in materials research, outlining three examples where a modern humidity controller has been successfully used to further understanding of how materials behave to develop optimized materials for a range of purposes.
Cure kinetics of silicone elastomers
Silicone sealants are used in several industrial, commercial, and domestic applications and are favored for their low modulus, electrical insulation, hydrophobicity, and low toxicity. Room-temperature vulcanizing (RTV) compounds are commonly used in silicone sealants which, once dispensed from an applicator, undergo a curing process and can be manipulated in situ until the cure is complete and the sealant is fully adhered to the substrate.
Most silicone RTV sealants rely on chemical reactions with water from the atmosphere to initiate and complete the curing process, and their cure kinetics under different environmental conditions have been the subject of research interest over many years. Studies into these so-called moisture-scavenging polymers have shown that temperature and RH have a significant impact on cure rates.1,2,3
Researchers at the University of Nottingham recently investigated the combined roles of temperature and humidity on the cure of an RTV silicone adhesive putty, known commercially as Sugru® and as Formerol® F10 (FormFormForm Ltd., UK) in industrial markets.4 Unlike many RTVs, this adhesive takes the form of a ductile solid in its pre-cured state, rather than a viscous fluid, making in situ shaping easier. It cures via condensation and hydrolysis reactions, where the initiation of cross-linking occurs upon exposure to atmospheric moisture.
The group measured the polymer’s cure progression via the shear modulus (measure of the elastic shear stiffness) as a function of time under controlled temperature and humidity conditions, to generate predictive models for cure timescales in relation to these environmental conditions. Conditions were controlled using a rheometer fitted with a sealed CTD450 environmental chamber fed by a humidity controller (Figure 1).
By coupling a humidity controller to a rheometer, characteristic cure timescales for a commercial and industrial silicone elastomer could be established under controlled environmental conditions. Results showed that, at constant absolute humidity, an increase in temperature from 19°C to 39°C reduced the curing timescale by approximately half (Figure 2). An increase in humidity from 7.6% to 36.7% RH (at constant temperature) reduced the cure timescale from approximately 11 hours to four hours (Figure 3). Understanding the cure behavior of such materials not only gives an indication of how fast the silicone will cure in different parts of the world where climates differ substantially, but can also be used to speed up or slow down cure rates where necessary (to increase molding time or solidify quicker). This is particularly important in a production environment where time savings translate into cost savings.
Energy storage properties of thermochemical materials
The technology that enables homes and businesses to run off renewable energy has advanced enormously in recent years to meet a growing demand for this energy source. To successfully harness solar energy, however, energy storage systems must be able to bridge the disconnect between supply and demand. Salt hydrates are a class of thermochemical material used to develop heat batteries that can supply low-temperature thermal energy in residential buildings during colder periods. Their high density and ability to store energy without significant losses make salt hydrates a popular material for heat batteries.
A reversible chemical reaction between the salt hydrate and water vapor governs this principle of energy storage, where the solid salt M combines with water vapor to initiate energy discharge:
M • aH2O(s) + (b-a) H2O(g) ⇌ M • bH2O(s) + Energy
The hydrated salt can be recharged by applying heat energy to the system, which converts the salt back to a lesser hydrated state and releases water vapor. Water vapor and temperature govern whether a salt is in a hydrated or dehydrated (anhydrous) state, and the hydration rate is defined as the extent of the transformation of a lower hydrated or dry salt into a higher hydrated state per unit of time. Repeatedly hydrating and dehydrating a salt is known as “cycling,” and there is significant research interest in the effects of repeated cycling on salt hydrate morphology and hydration rate.
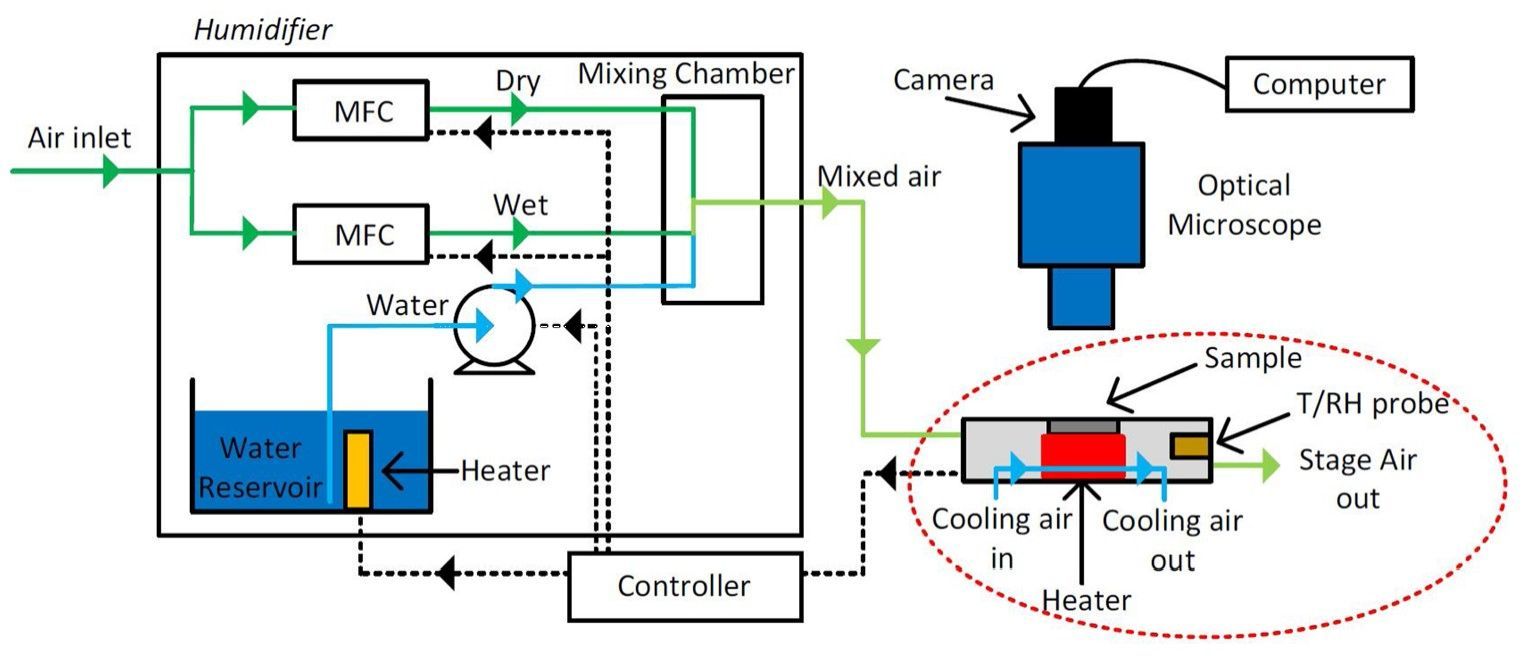
A group at the Eindhoven University of Technology investigated changes in particle size and crack formation of potassium carbonate (K2CO3) particles in a microclimate chamber using optical microscopy.5 The researchers hypothesized that crack formation over repeated cycles increases the hydration rate of the salt hydrate, as the expansion and breaking of the particle increases its microporosity and promotes water transport.
The microclimate chamber, also known as a “hot-stage” was connected to a humidity controller which enabled precise control over the experimental temperature and RH (Figure 4). The hydration rate of the K2CO3 particles was evaluated by thermo gravimetric analysis (TGA), through simultaneous thermal analysis and the group developed a nucleation and growth model to describe the salt’s solid-state reactions, which incorporated crack formation.
K2CO3 particles underwent 12 cycles in the hot-stage and their size was measured after each dehydration step, with an image being captured every 10 minutes by the optical microscope to show the “apparent area” (Figure 5). The particles were found to increase in size by roughly 30 percent over the 12 cycles. This increase was attributed to the cracking of individual crystals, confirming the hypothesis that cycling K2CO3 particles increases their size through crack formation. TGA experiments revealed that cycling K2CO3 particles significantly reduces the hydration rate, from approximately 8.3 hours for the first cycle to 33 minutes for the 12th cycle. Together with the humidity- and temperature-controlled microscopy experiments, these results confirm the observation that an increase in salt hydrate particle size leads to a faster rate of hydration.
Extending the lifetime of solar cells
The effect of temperature and humidity on another renewable energy technology—solar cells, or photovoltaics (PVs)—is gaining increasing attention in the materials research community. Perovskite PVs have undergone rapid improvements in recent years and demonstrated considerable power conversion efficiency. Perovskite PVs have a classic layered structure composed of a transparent conducting oxide, a hole transport layer, a perovskite active layer, an electron transport layer, and an electrode such as gold (Au) or aluminum (Al).
However, their stability and durability represent a significant challenge in developing these devices for commercialization. According to the International Electrotechnical Commission's (IEC) standards, solar cells must perform well under non-laboratory conditions, such as in damp conditions (i.e. 85% humidity at 85°C) for more than 1,000 hours consistently.6 Precise control of temperature and humidity in perovskite PV research and development that takes into account the multiple layered structure is therefore critical.
One study used temperature and humidity control devices with in situ Raman spectroscopy to investigate perovskite PV degradation mechanisms, to better understand the role of these environmental conditions on degradation kinetics.7 Raman spectroscopy also allows researchers to examine the degradation of individual perovskite layers. Results from the in situ Raman humidity experiments showed that the dihydration of perovskite is almost completely reversible once drying occurs (Figure 6). Deeper analysis revealed that dihydration remained at the Au region, indicating that some moisture remained trapped in this region. It was deduced that PV device performance could be fully recovered if the trapped moisture could be removed.
Increasing the bond strength between the organic component of perovskite PVs and the metal halides could reduce the effect of potentially irreversible damage to the device due to trapped moisture,8 and hydrophobic interlayers can also be introduced to help protect the perovskite from ambient moisture.9 Experiments that can control humidity and measure its impact on different layers within perovskite PVs will be vital in the efforts to develop this type of solar cell for market.
Future of humidity control
The examples presented in this article provide a small snapshot of the huge number of applications benefiting from humidity and temperature control devices. These devices, which can be paired with a wide range of analytical methods, help researchers gain a deeper understanding of how materials behave under various environmental conditions. Recent advances in humidity control technology offer superior sensitivity and precision to accurately replicate the environmental conditions that a material could be subject to.
References:
- Halasz L and Belina K, An investigation into the curing of epoxy powder coating systems, J. Therm. Anal. Calorim. 119 (2015) 1971–1980.
- Hong I and Lee S, Cure kinetics and modeling the reaction of silicone rubber, J. Ind. Eng. Chem. 19 (2013) 42–47.
- Comyn J, Moisture cure of adhesives and sealants, Int. J. Adhesion Adhes. 18 (1998) 247–253.
- Elsmore MT and De Focatiis DSA, Combined roles of temperature and humidity on cure of a silicone elastomer, Polymer Testing, 93 (2021) 106967.
- Beving MAJM, Frijns AJH, Rindt CCM, and Smeulders DMJ, Effect of cycle-induced crack formation on the hydration behaviour of K2CO3 particles: Experiments and modelling. Thermochimica Acta, 692 (2020) 178752.
- International Standard IEC61215. Terrestrial Photovoltaic (PV) Modules—Design Qualification and Type Approval—Part 1: Test Requirements and—Part 2: Test Procedures, 1.0 ed.; TC 82—Solar Photovoltaic Energy Systems; IEC: Geneva, Switzerland, 9 March 2016.
- Hooper KEA, Lee HKH, Newman ML, et al. Probing the degradation and homogeneity of embedded perovskite semiconducting layers in photovoltaic devices by Raman spectroscopy, Phys. Chem. Chem. Phys., (2017) 19, 5246.
- O’Kane M, Perovskite Solar Cells: Causes of Degradation, Ossila Ltd. https://www.ossila.com/pages/perovskite-solar-cell-degradation-causes [Accessed 16/08/2021]
- Rajagopal A, Yao K, and Jen AKY, Towards Perovskite Solar Cell Commercialization: A Perspective and Research Roadmap Based on Interfacial Engineering, Advanced Materials (2018) 30(32): 1800455.