In the biopharmaceutical field, mycoplasma contamination acts like an invisible killer, constantly threatening drug safety and patient health. As the industry evolves rapidly, mycoplasma testing methods are no longer sufficient to meet the timeliness requirements of modern drug production. The emergence of rapid release testing technologies has opened a new door for the biopharmaceutical industry—one that is both safe and highly efficient. This technology not only shortens the detection cycle but also provides a powerful safeguard for biologics quality control.
Mycoplasma detection: The safety cornerstone of biopharmaceuticals
Hazards of mycoplasma
As the smallest self-replicating prokaryotes, mycoplasmas can cause abnormal cell growth, metabolic disruption, or even apoptosis, thereby endangering patient health. For instance, Mycoplasma pneumoniae infection often leads to pneumonia, with symptoms such as persistent fever, dry cough, and chest pain, which can escalate to respiratory distress and complications. In manufacturing, mycoplasma contamination may stem from raw materials, personnel, or the lab environment, and poses hidden, persistent risks—necessitating strict monitoring at every stage of the production process.
Limitations of detection methods
Culture-based detection methods take 28 days to yield results, severely limiting product release speed. While PCR has reduced testing time, it still carries risks of false positives/negatives and does not fully meet the demands for rapid and accurate modern drug testing.
Rapid release testing: An innovative solution for mycoplasma detection
By integrating advanced technologies such as nucleic acid amplification and fluorescence detection, rapid release testing achieves highly sensitive detection of mycoplasma DNA. This method shortens testing cycles to just hours while maintaining precision and reliability. It detects ultra-low levels of contamination and provides a solid basis for quality assessment.
The adoption of rapid testing has shortened biologics release times from weeks to days, significantly boosting manufacturing efficiency and reducing batch failure risks—saving companies substantial costs. In the age of precision medicine, this technology plays a vital role in emerging areas like cell and gene therapy. Its continued innovation is propelling the biopharma QC framework to new heights, ensuring safer and more effective treatments for patients.
Accurate workflow: ACROBiosystems supports robust quality control
Sample preparation
Sample preparation is critical in rapid release testing. Optimized lysis buffers and nucleic acid extraction solutions maximize DNA recovery while removing PCR inhibitors. The use of automated nucleic acid extraction instruments is especially recommended, as it further enhances the consistency and reliability of sample processing.
Standardization of detection methods
Standardization ensures accuracy. Validated primer-probe combinations and optimized reaction systems minimize inter-batch variability. Real-time qPCR allows real-time monitoring, enhancing accuracy and reproducibility.
ACROBiosystems’ mycoplasma DNA sample preparation kit (Cat. No. OPA-E101) and mycoplasma detection kits (Cat. No. OPA-S102) comply with pharmacopoeial testing requirements in multiple regions, including China, the U.S., and Europe. They are supported by third-party validation reports from Eurofins. The qPCR detection performance is consistent with culture methods and indicator cell assays, covering all strains required by the aforementioned pharmacopeias. In addition, human mycoplasma has been specifically validated, providing more comprehensive scientific evidence for detection efficacy.
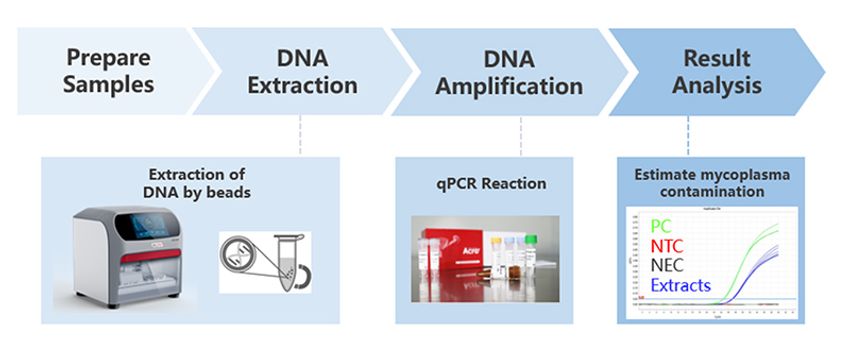
Figure 1. ACROBiosystems offers a comprehensive mycoplasma detection solution.
CREDIT: ACROBiosystems
Full-process QC optimization & support
Establishing a QC system involves staff training, equipment calibration, and reagent validation. Standard operating procedures and strict process control ensure traceability. ACROBiosystems’ technical team supports platform setup, workflow optimization, validation planning, and regulatory submissions—saving time and cost through turnkey solutions.
Validation data
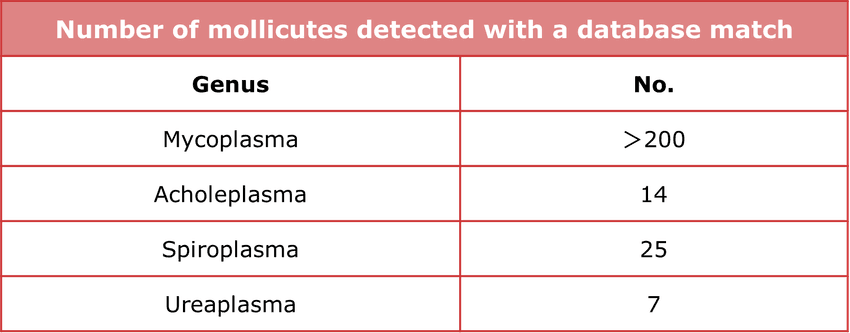
Table 1. Broad coverage. Detects over 250 species of mycoplasma and mollicutes.
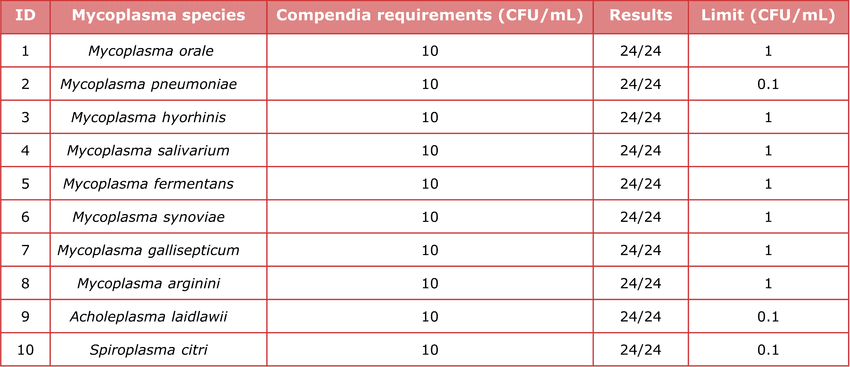
Table 2. High sensitivity. Tested 10 mycoplasma standards (10 CFU/mL) in 24 replicates each; all returned positive—meeting/exceeding EP 2.6.7 regulatory standards (10 CFU/mL).
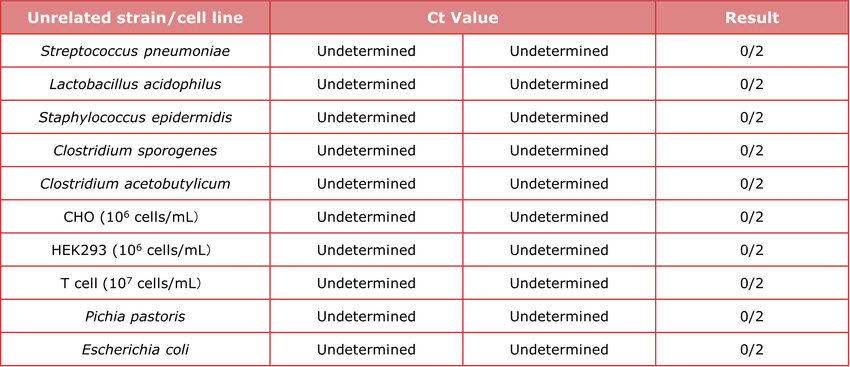
Table 3. High specificity and no cross-reactivity. Tested against two cell lines and four unrelated bacteria (e.g., Streptococcus pneumoniae, Lactobacillus acidophilus, Staphylococcus epidermidis, Bacillus subtilis)—all negative, confirming no cross-reactivity.
References
U.S. Food and Drug Administration. 2018. “Elimination of 21 CFR 610.30 Test for Mycoplasma.”
Marians, R. C., Brenton, M., LaBombard, S., et al. (2014). “Regulatory Aspects of Mycoplasma Testing for Cell Therapy Products.” LABS, Inc.

CREDIT: ACROBiosystems





