In a world focused on pandemic response, including disease treatment and pharmaceutical production, not to mention genetic and cancer research, biosafety level-rated labs are critical. Specialized containment labs are also increasingly necessary for clinical, diagnostic, production, and research facilities.
Having worked as the “safety guy” at a large public university that included a medical school, dental school, veterinary school, animal research colonies, biomedical and cancer research facilities, and a nanotechnology research facility, I was presented with every type of containment laboratory and cleanroom. One thing they all had in common was the need to properly gown up prior to entry and de-gown before exiting.
The specific personal protective equipment (PPE) required strictly depends on the type of containment area and the ongoing research. This article provides a generic procedure that can be tailored to fit most any containment lab or cleanroom, both sterile and non-sterile.
Containment lab general setup
Containment laboratories are constructed so that the entire room is a secondary containment barrier.1 That is, the lab space ventilation is kept pressurized slightly negative relative to the adjacent areas. This is to maintain an inward directional airflow by exhausting more air than is supplied. Thus, any contaminants from spills or releases are prevented from migrating into surrounding areas. The laboratory exhaust must vent directly to the outside air with no recirculation. Depending on the research and materials in use, in most cases, the exhaust air must also be filtered, usually with high efficiency particulate air (HEPA) filters.1
Ideally, separate areas are provided for entry and gowning-in versus de-gowning and exit, although many facilities use a single access for entry and exit. Whether one-directional or not, the ingress/egress point(s) should use a two-stage process: a pregowning area where the process is started followed by the gowning or PPE donning room. In a model facility, exit is via a separate de-gowning room proceeding then to final clearance and exit. Airflow is strictly controlled in these areas to fully contain any contaminants.
Pre-gowning precautions
Prior to beginning the process of entering or using a cleanroom or containment lab, ensure your standard operating procedures (SOPs) and protocols are clearly and concisely written and all employees (entrants) are thoroughly trained. Your written SOP should:
- Minimize the use of make-up, hair gel, body lotions, and personal skin care products as these can potentially introduce contaminants.
- Users should not smoke within 45 minutes of entering, especially cleanrooms, as it is well-documented that smokers shed particulates for much longer than 30 minutes after smoking.
- Remove extraneous street clothing such as sunglasses, hats, jackets, etc. before entering the antechamber to simplify the process and minimize needed actions.
- Plan out the work in advance so all materials, tools, solutions, etc. are on hand and ready to minimize traffic and the number of entries/exits.
A recommended gowning procedure
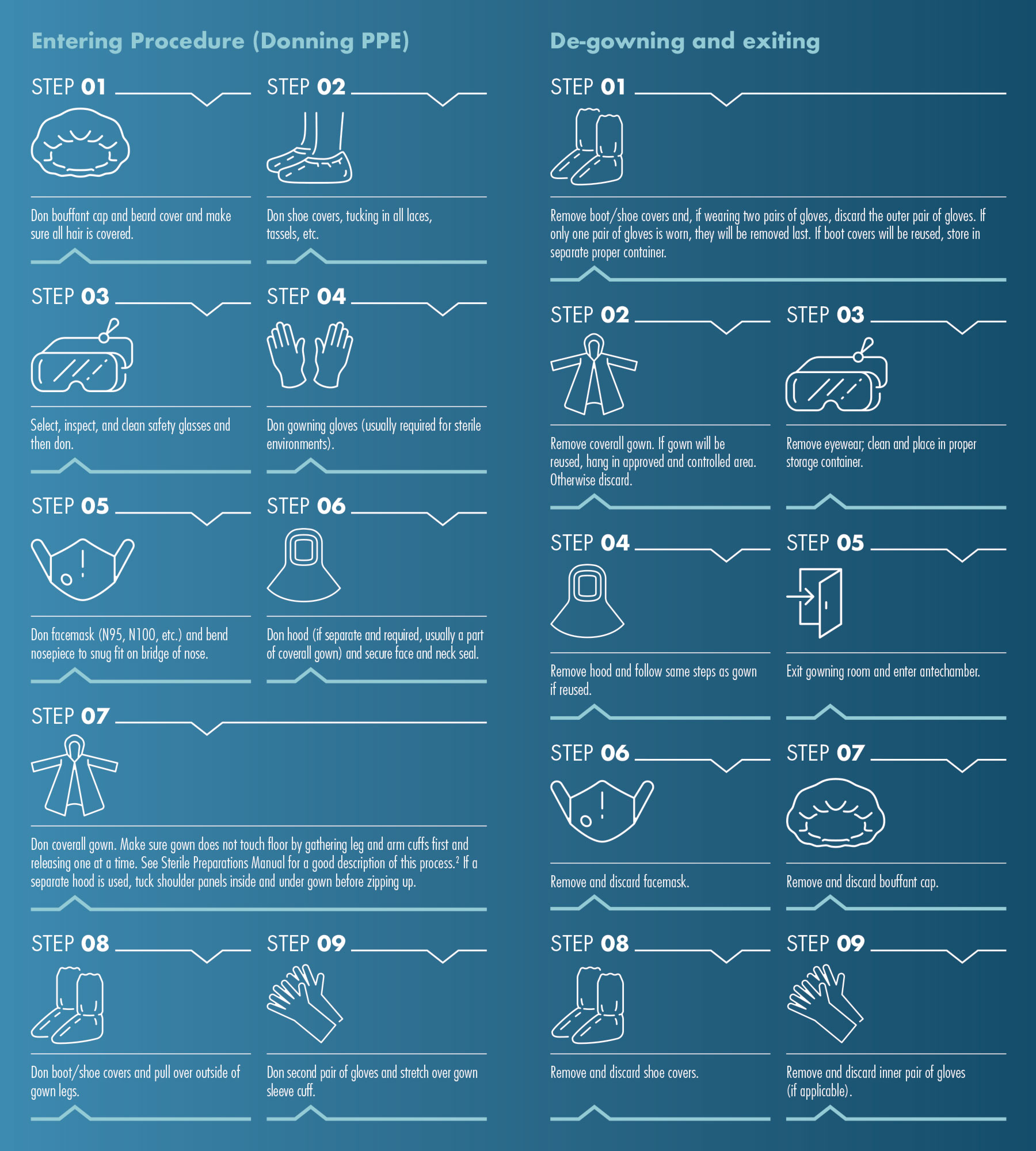
The following graphic demonstrates the procedure for a basic level containment lab or cleanroom and provides a generic order for donning PPE items. The sequence is designed to help control contamination when donning and removing standard containment/cleanroom PPE. Not every apparel article is needed in all cases. Check your facility’s procedures. If you are dealing with highly infectious or toxic agents or working in a highly sterile lab (pharmaceutical preparations, FDA regulated lab, etc.), then additional steps and much stricter protocols will be necessary.2
After following the steps outlined in the “Donning PPE” section of the graphic, you are ready to enter the cleanroom or containment lab. Upon completion of your work, exiting the containment area is generally the reverse of those steps. However, there are important considerations, so a list of the full steps is also featured in the graphic.
Work in containment labs and cleanrooms is always serious business, especially so during a pandemic. Failure to follow protocols could potentially put you, your coworkers, and others in danger or at risk. Contamination could cause personnel illness, loss of many hours of research, and possible huge financial losses. The PPE requirements are put in place for critical and specific reasons. Be patient and properly gown in and out every time you enter a containment area. And remember—safety first!
References:
- Biosafety in Microbiological and Biomedical Laboratories, 5th Edition, Center for Disease Control, US Department of Health. Atlanta, GA. October 2018. http://www.cdc.gov/biosafety/publications/bmbl5/
- Compounding Sterile Preparations. American Society of Health-System Pharmacists. 2014. https://www.ashp.org/-/media/assets/policy-guidelines/docs/guidelines/compounding-sterile-preparations.ashx