Application note | CTS Xenon Electroporation System
Flexible and scalable nonviral delivery for cGMP cell therapy processing
Scale and optimize your transfection in a closed system with the CTS Xenon Electroporation System
Introduction
Closed manufacturing of cell therapies is widely accepted as an effective way to create a safer therapeutic product through reduction of error often associated with traditional manual cell processing techniques. In addition to closing manufacturing systems, the industry is moving toward targeted gene delivery methods such as transfection with CRISPR-Cas9 to achieve both performance and safety enhancements over traditional random integration of vectors [1].
Thermo Fisher Scientific is developing a family of highly flexible and modular instruments that can be digitally and physically connected to create a closed, end-to-end cell therapy manufacturing platform. Here, we introduce the Gibco™ CTS™ Xenon™ Electroporation System and highlight its ability to transform your current transfection step into one that’s closed, optimized, and scalable. To demonstrate a transition from research and discovery to process development and manufacturing, we compare the CTS Xenon system to the Invitrogen™ Neon™ Transfection System, a small-scale, benchtop device that is suitable for research and early process development.
Key features of the CTS Xenon Electroporation System
Scalability—consistent performance across various volumes (1 mL and 5–25 mL) allows for optimization at small-scale parameters and seamless transition to larger scale applications
High speed, large volume—transfect up to 2.5 x 10? T cells in less than 25 minutes
Proven performance and viability—up to 90% gene knockout and 80% viability
Process flexibility—user-programmable system enables you to create and optimize electroporation protocols for various cell types and payloads, from process development through commercial manufacturing
Efficient nonviral transfection—can be used to deliver DNA, RNA, and protein payloads
Closed-system processing—sterile, single-use Gibco™ Xenon™ MultiShot Electroporation Cartridge enables sterile welding to PVC or C-Flex™ tubing
Materials and methods
Overview
Peripheral blood mononuclear cells (PBMCs) were previously isolated from an apheresis product using the Gibco™ CTS™ Rotea™ Counterflow Centrifugation System and frozen for future use (Figure 1). On day 0, PBMCs from three different donors were thawed
and activated using Gibco™ CTS™ Dynabeads™ CD3/28 and expanded in Gibco™ CTS™ OpTmizer™ T Cell Expansion SFM, supplemented with Gibco™ CTS™ Immune Cell SR and other components as instructed per product insert. On day 3, activated T cells were debeaded and electroporated using either the Neon Transfection System or the CTS Xenon Electroporation System in conjunction with Invitrogen™ TrueCut™ Cas9 Protein v2; the cells were put back into culture for expansion in complete CTS OpTmizer medium.
On day 6, cells were analyzed for viability, phenotype, as well as knock-in and knockout efficiency with the Invitrogen™ Attune™ NxT Flow Cytometer.
Figure 1. Autologous T cell therapy workflow.
Electroporation experimental setup
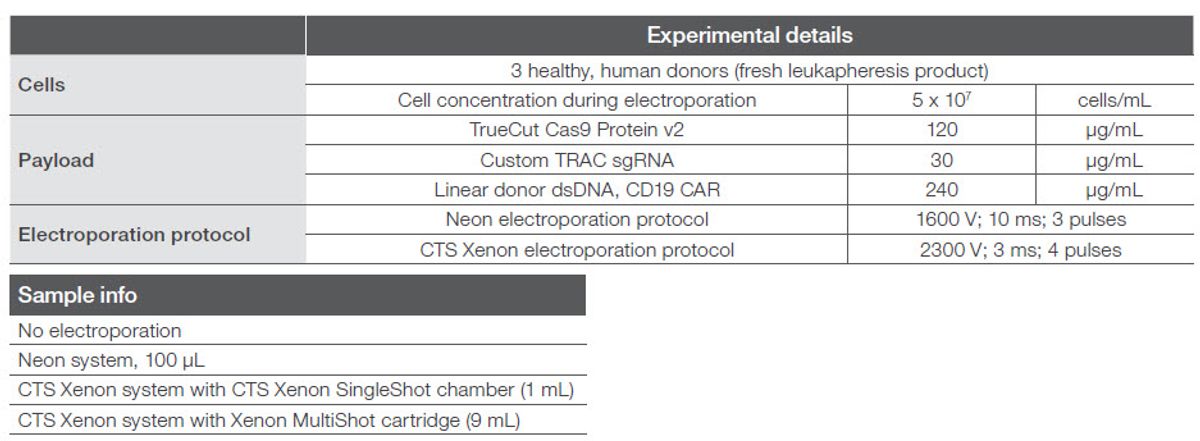
To demonstrate the reproducibility across different electroporation scales, we tested three different electroporation volumes under comparable electroporation conditions, across three different donors, one run per donor. Electroporation volumes tested were 100 µL on the Neon system, 1 mL on the CTS
Xenon system using the Gibco™ CTS™ Xenon™ SingleShot Electroporation Chamber, and 9 mL on the CTS Xenon system using the Xenon MultiShot cartridge, with a cell density of 5 x 107 cells/mL in Gibco™ CTS™ Xenon™ Genome Editing Buffer across all donors and scale conditions. A control for no electroporation was also tested. To demonstrate a clinically relevant genetic modification strategy (Figure 2), we utilized the Neon and CTS Xenon electroporation systems to deliver TrueCut Cas9 Protein v2 and TRAC-encoded Invitrogen™ TrueGuide™ Synthetic gRNA (sgRNA) along with a donor dsDNA chimeric antigen receptor (CAR) construct. Respective payload quantities and electroporation conditions utilized are listed in Figure 2.
Figure 2. Experimental conditions and electroporation parameters.
Flow cytometry gating strategy
The Attune NxT Flow Cytometer was utilized for assessment of gene editing efficiency and phenotype, and Figure 3 shows the gating strategies applied.
Figure 3. Gating strategy for the Attune NxT Flow Cytometer.
Results Viability
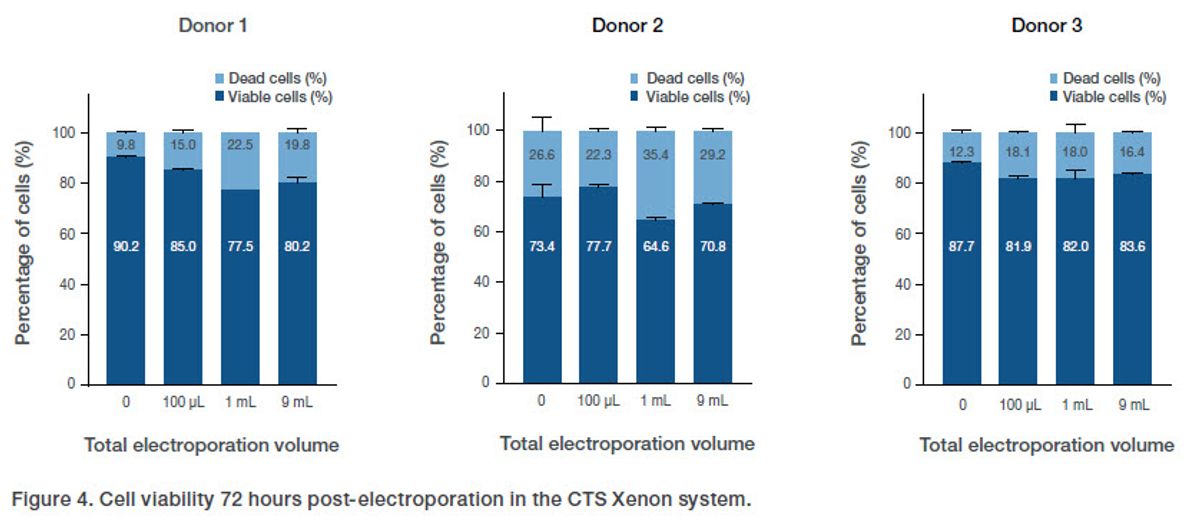
Electroporation success is a balance between transfection efficiency and viability, and, generally, greater than 70% viability is acceptable post-electroporation. Viability was assessed 72 hours post-electroporation and was consistent across various electroporation volume scales including the no electroporation control, showing that electroporation itself and the electroporation volume did not significantly alter cell viability (Figure 4). In addition, viability in the CTS Xenon system was well above 70% with the exception of donor 2, which had poor starting viability.
Figure 4. Cell viability 72 hours post-electroporation in the CTS Xenon system.
Transfection efficiency
Ribonucleoprotein complexes comprising TrueCut Cas9 Protein v2 and TrueGuide sgRNA encoding for the TRAC site were used in conjunction with a dsDNA CAR construct to genetically modify activated T cells utilizing either the Neon (100 µL) or CTS Xenon (1 and 9 mL) transfection systems. Figure 5 showcases consistent gene editing efficiency; at times, superior efficiency was observed with the CTS Xenon system (22–46% knock-in efficiency) compared to the Neon system (12–38% knock-in efficiency), suggesting that the CTS Xenon system can be used to easily scale and optimize the transfection process in a closed system.
Figure 5. Gene editing efficiency. The dark blue bar represents the percent of cells expressing the integrated CAR sequence (knock-in), light blue represents knockout, and grey represents the unedited or wild type population.
Phenotype
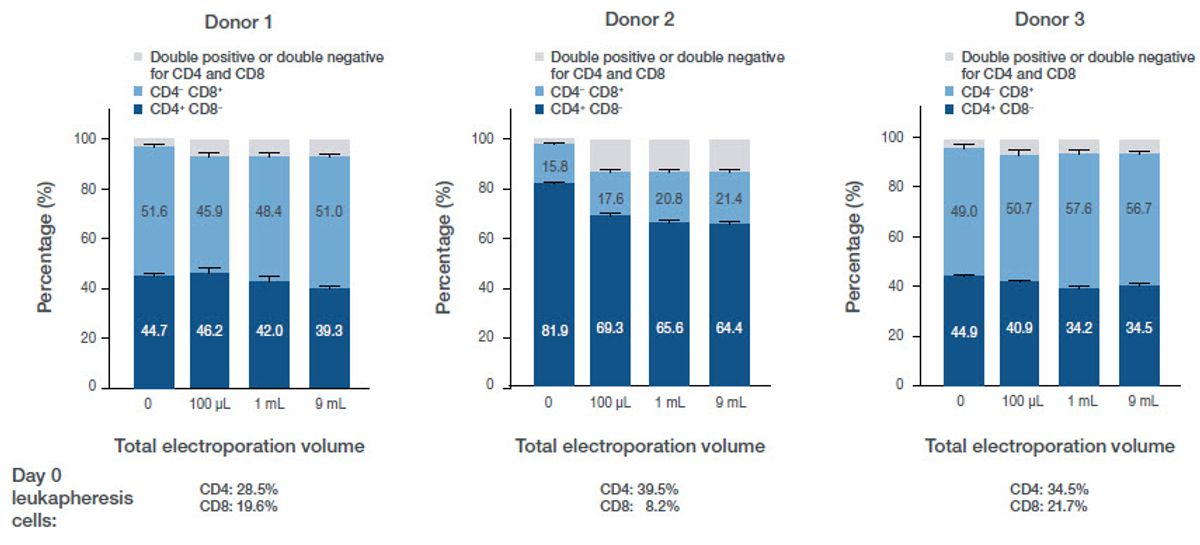
T cell phenotype was assessed on the Attune NxT Flow Cytometer. Compared to nonelectroporated controls, there is minimal or no phenotypic change across the electroporation volumes tested. There was a shift in phenotype when comparing cells before and after activation, electroporation, and expansion, but no significant impact from electroporation alone (Figure 6).
Figure 6. Phenotypic data post-electroporation. The dark blue bar represents CD4? cells, the light blue bar represents CD8? cells, and the grey bar represents either double positive or double negative CD4/8 cells. Below the bar charts is phenotypic characterization before activation, electroporation, and culture.
Discussion
To achieve the ease in scale-up as demonstrated here, and deliver efficiency in process development, the CTS Xenon system needed to have an equivalent pulse profile, scalable electroporation protocols, and equivalent or superior performance compared to the Neon system.
Unlike standard cuvette-based chambers, the Neon system uses a patented biologically compatible tip chamber that generates a more uniform electric field and exposes the sample to a smaller surface area of the metal during electroporation, both of which help the Neon system provide
a high level of transfection efficiency and viability. In an effort to deliver performance at scale without requiring the user to reoptimize electroporation parameters, we set stringent hardware and performance requirements for the large-scale system to be equivalent to the 100 µL tip of the Neon system.
Although reoptimization to transition protocols from the Neon system to the CTS Xenon system is not generally required, flexibility and tailoring of electroporation parameters are still critical in identifying optimal protocol conditions. The CTS Xenon system allows for the same optimization and manipulation of parameters as the Neon system—voltage, pulse width, and pulse number—
with an added parameter of pulse interval, which is the time (delay) between pulses in a multi-pulse electroporation profile. This parameter can be reduced two-fold in the CTS Xenon system to reduce the processing time required for large volume electroporation runs. Finally, the data presented here demonstrate the ability of the CTS Xenon system to deliver consistently high viability, transfection efficiency, and minimal change in phenotype.
Conclusions
As demonstrated through scale-up from the Neon system to the CTS Xenon system, the CTS Xenon Electroporation System enables transition from research to commercial manufacturing by facilitating scalable and flexible nonviral delivery for cGMP cell therapy processing.
Large-volume electroporation system designed for cell therapy process development and manufacturing |
|
|
|
|
Instrument | Electroporation chamber | Electroporation buffers | Software |
Designed for effcient process development and improved quality in GMP manufacturing Easily integrates into existing processes CTS product manufactured according to GMPs
| | Two buffers: electroporation and genome editing Available in 100 mL bottles and 100 mL bags CTS product manufactured according to GMPs
| Intuitive, easy-to-use eGUI (embedded graphical user interface) Available software upgrade that helps enable compliance with 21 CFR Part 11
|
Facilitates effcient scale-up from small to large volume; helps improve quality in GMP manufacturing |
Thermo Fisher can supply Regulatory Support Files for both the instrument and consumables to assist in regulatory filings. It is the responsibility of the end user to ensure regulatory compliance.
Ordering information |
|
Description | Quantity | Cat. No. |
Nonviral genetic modification |
|
|
CTS Xenon Electroporation System | 1 ea | A52727 |
CTS Xenon SingleShot Electroporation Chamber, 1 mL | 6 pk | A53444 |
Xenon MultiShot Electroporation Cartridge, 5–25 mL (coming soon) | 1 pk | A53445 |
CTS Xenon Genome Editing Buffer, bottle | 100 mL | A4998001 |
CTS Xenon Genome Editing Buffer, bag | 100 mL | A4998002 |
TrueCut Cas9 Protein v2 | 100 µg | A36498 |
Custom TRAC-encoded TrueGuide sgRNA | Custom | Please inquire |
T cell culture and activation |
|
|
CTS OpTmizer T Cell Expansion SFM, bottle | 1,000 mL | A1048501 |
CTS OpTmizer T Cell Expansion SFM, bag | 1,000 mL | A1048503 |
CTS Immune Cell Serum Replacement (ICSR) | 50 mL | A2596101 |
CTS GlutaMAX-I Supplement | 100 mL | A1286001 |
CTS GlutaMAX Supplement, bag | 100 mL | A4737001 |
Human IL2 (Interleukin 2) Recombinant Protein | 1 mg | PHC0023 |
CTS Dynabeads CD3/CD28 | 10 mL | 40203D |
T cell analysis |
|
|
Attune NxT Flow Cytometer | 1 ea | A29004 |
Attune NxT Flow Cytometer Autosampler | 1 ea | 4473928 |
V5 Tag Monoclonal Antibody (TCM5), PE | 100 tests | 12-6796-42 |
CD4 Monoclonal Antibody (RPA-T4), PE-Cyanine7 | 100 tests | 25-0049-42 |
CD8 Monoclonal Antibody (RFT-8), APC-Cyanine7 | 100 µg | A15448 |
SYTOX Red Dead Cell Stain, for 633 or 635 nm excitation | 1 mL | S34859 |
eBioscience Flow Cytometry Staining Buffer | 200 mL | 00-4222-57 |
References
Roth TL et al. (2018) Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559(7714): 405-409.
Eyquem J et al. (2017) Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543(7643): 113-117.
Learn more at thermofisher.com/xenon
Intended use of the products mentioned in this document varies. For specific intended use statements, please refer to the Instructions for Use (IFU). © 2022 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. C-Flex is a trademark of
Saint-Gobain Performance Plastics Corporation. COL34752 0322