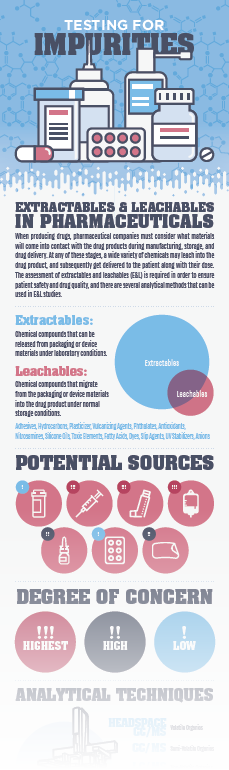
When producing drugs, pharmaceutical companies must consider what materials will come into contact with the drug products during manufacturing, storage, and drug delivery. At any of these stages, a wide variety of chemicals may leach into the drug product, and subsequently, get delivered to the patient along with their dose.
The assessment of extractables and leachables (E&L) is required in order to ensure patient safety and drug quality, and there are several analytical methods that can be used in E&L studies.
Download this free infographic and learn:
- more about chemical compounds that can be released from packaging or processing equipment
- potential sources of contamination
- analytical techniques for identification and quantification