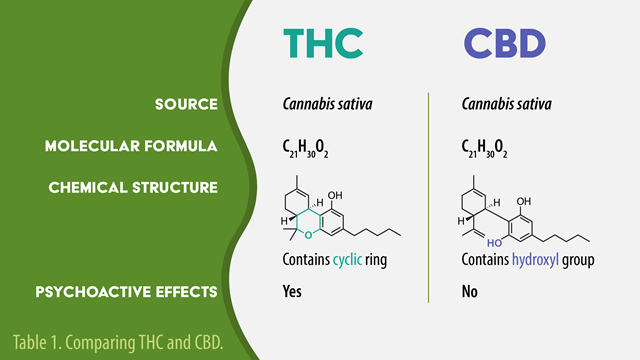
Cannabis is a product of the cannabis sativa plant, and contains many cannabinoids that interact with the endocannabinoid system. Delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) are two of the most well-studied cannabinoids. THC is psychotropic, and capable of producing a “high,” while CBD is non-psychotropic and does not exert this effect.
Biosynthesis of THC vs CBD
Cannabigerolic acid (CBGA) is the precursor to both THC and CBD. Cannabidiolic synthase (CBDA synthase) and tetrahydrocannabinolic acid synthase (THCA synthase) catalyze the oxidative cyclization of CBGA into CBDA and THCA, respectively. CBD and THC are produced by subsequent decarboxylation of these acidic forms.

The mechanism of action of THC vs CBD
THC exerts its effects via interaction with the CB1R (endocannabinoid type 1 receptor) and CB2R (endocannabinoid type 2 receptor). CB1Rs are abundant in the brain, located in regions associated with cognition, memory, reward, and pain perception, among others, and CB2R are mostly found in the periphery, on immune cells, lymphoid tissue, and peripheral nerve terminals.
CB1R and CB2R interact with endogenous ligands including anandamides, which are arachidonic acid derivatives. The endogenous system modulates neuronal activity via cyclic-AMP, Ca2+ transport, and K+ ions, and is thought to be associated with opioid, GABAergic, dopaminergic, and several other systems. THC exerts its psychotropic effects via CB1R binding and downstream signaling.
Alternatively, CBD does not bind and stimulate the CB1 receptor, and therefore does not exert psychotropic effects. Recent evidence suggests CBD is a negative allosteric modulator of CB1R, preventing THC binding and stimulation. It has also been suggested that CBD may interact with the endocannabinoid system via indirect mechanisms including: inhibition of adenosine uptake, enhanced 5-HT1A receptor activity, enhanced activity at glycine receptor subtypes, among others.

THC for medical conditions
There is significant anecdotal evidence supporting the use of cannabis for a variety of medical conditions, however, there is limited literature to support these claims. Many studies are underway to examine the safety and efficacy of cannabis for the treatment of various medical conditions including:
- Inflammation
- Pain
- Seizure
- Mental disorders including depression and anxiety
- Dermatologic conditions
- Cancer pain management
- Neurodegenerative disorders including: Alzheimer’s disease, Huntington’s disease, Parkinson’s disease
- Migraine
Randomized controlled trials investigating THC
RCTs investigating the effects of cannabis are limited, due in part to the legality of its use. However, there have been some randomized controlled trials examining the efficacy of smoked cannabis for various medical conditions:
- Cannabis has been shown to relieve chronic neuropathic pain among patients with HIV-associated sensory neuropathy.
- Cannabis has been shown to be superior to placebo for relieve of treatment-resistant spasticity among patients with multiple sclerosis.
- Cannabis has been shown to lower intraocular pressure in patients with heterogenous glaucoma.
- Cannabis has been shown to ameliorate neuropathic pain.
THC risks and side effects
There are some risks and side effects associated with both short-term and long-term cannabis use. Short-term effects include impaired short-term memory, motor coordination (impairing driving ability), altered judgement, and when consumed in high doses, paranoia and psychosis. Long-term or heavy use is associated with altered brain development especially among those consuming cannabis in adolescence. It may also increase the risk of developing chronic psychosis disorders such as schizophrenia among predisposed individuals.
There are also some possible side effects associated with medical cannabis use:
- Anxiety
- Dizziness
- Drowsiness
- Nausea and vomiting (sometimes severe)
- Lung infections (if smoked)
- Dry mouth
CBD for medical conditions
As CBD does not produce a high, and does not associated with abuse or dependence potential, it is an appealing option for managing many medical conditions. Epidiolex, is a CBD oral solution that has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of seizures associated with rare and severe forms of epilepsy (Lennox-Gastaut syndrome and Dravet syndrome) in young children. There are also many clinical trials underway to assess the effects of CBD for various medical conditions, including:
- CBD for reducing tremor among patients with Parkinson’s disease.
- CBD for reducing disease activity among patients with inflammatory bowel disease (IBD).
- Effect of CBD as a treatment intervention for opioid relapse
- Effect of CBD on responses to emotional stimuli (to examine the potential effect of CBD as an anxiety-reducing drug)
Randomized controlled trials investigating CBD
Several RCTs investigating the effects of CBD have been completed, including:
- CBD for acute schizophrenia
- CBD for generalized anxiety disorder
- CBD for seizures in tuberous sclerosis complex
CBD risks and side effects
The FDA does not currently regulate the safety or purity of CBD sold as a dietary supplement, and it may contain other unknown ingredients. The therapeutic dose of CBD for specific medical conditions has also not been established. Some common side effects associated with CBD consumption include:
- Nausea
- Fatigue
- Irritability
- CBD may also elevate the concentration of certain medications via a similar mechanism to grapefruit juice













