Julianne L. Baron, PhD, CPH, RBP is the president of Science and Safety Consulting. She has a background in infectious diseases, biosafety, and public health and consults on laboratory safety and design, pandemic preparedness, and scientific communication.

Julianne Baron
Credit: Julianne Baron
Routine surface decontamination of your biosafety cabinet (BSC) is industry best practice before and after work with biological materials, however, there are some instances when a full space decontamination may be necessary. In this article, we will discuss situations when a space decontamination is warranted, what chemical sterilants are available for fumigation of biosafety cabinets, how to prepare for this kind of decontamination, and design features of BSCs that may help facilitate decontamination of the entire BSC using these chemicals.
Reasons to consider gaseous decontamination of your biosafety cabinet
During normal use of your BSC, surface decontamination should be performed regularly including before and after work, if product contamination is attributed to the BSC, and commonly using a modified decontamination protocol after spills of biological materials. For more information about surface decontamination of BSCs, please review the following resources.1-4
Surface decontamination, however, may not be sufficient for all situations, such as those where the internal or physically unreachable surfaces including HEPA filters, motor blowers, and air plenums of the BSC need to be decontaminated.5 Certain chemical sterilants may be used in their vapor or gaseous forms to thoroughly spread throughout and fumigate all internal surfaces and components of BSCs, and other laboratory spaces like rooms, suites, furniture, and different laboratory equipment.3-6 Treatment with chemicals in vapor or gas form, collectively referred to as “gas decontamination”, is done to take advantage of the distribution and penetration ability of gases.3

Class II Type A2 Biosafety Cabinet
Credit: NuAire
A risk assessment or specific regulations may dictate when a BSC should be subjected to gas decontamination, but industry standards and guidance documents also list certain instances when this type of decontamination should be performed, including:
• Before changing the HEPA filter(s)
• Before performing maintenance, repair, or replacement of internal BSC components
• Before field certification of BSCs used in BSL-2 (gas decontamination is desirable), BSL-3 (gas decontamination is recommended) or BSL-4 (gas decontamination is required) laboratories
• Before removal of potentially contaminated equipment from inside the BSC
• Before moving the BSC to another location
• Before significant changes in the agents used within the BSC
• After significant spills or splashes if surface decontamination is thought to be insufficient
• Before final decommissioning, salvage, or disposal of the BSC3-6
Features of commonly used chemicals for BSC gas decontamination
There are three chemical sterilants listed in the NSF/ANSI Standard 49 (2022) and most biosafety guidance documents for gas decontamination of biosafety cabinets and larger laboratory spaces and equipment. These treatment methods include the use of both gas (formaldehyde and chlorine dioxide) and vapor (hydrogen peroxide) formulations of chemical sterilants.3-6 The NSF/ANSI Standard 49 notes that the chemicals used in this decontamination process always should be EPA registered pesticides.5 A risk assessment is necessary to determine which chemical would be most appropriate for the gas decontamination of a particular BSC. This process typically involves the BSC user, safety professionals, and the company that has been hired to perform the gas decontamination. There are many features to review when choosing the appropriate chemical sterilant, especially if the biosafety cabinet is going to be gas decontaminated repeatedly or frequently during its lifetime.
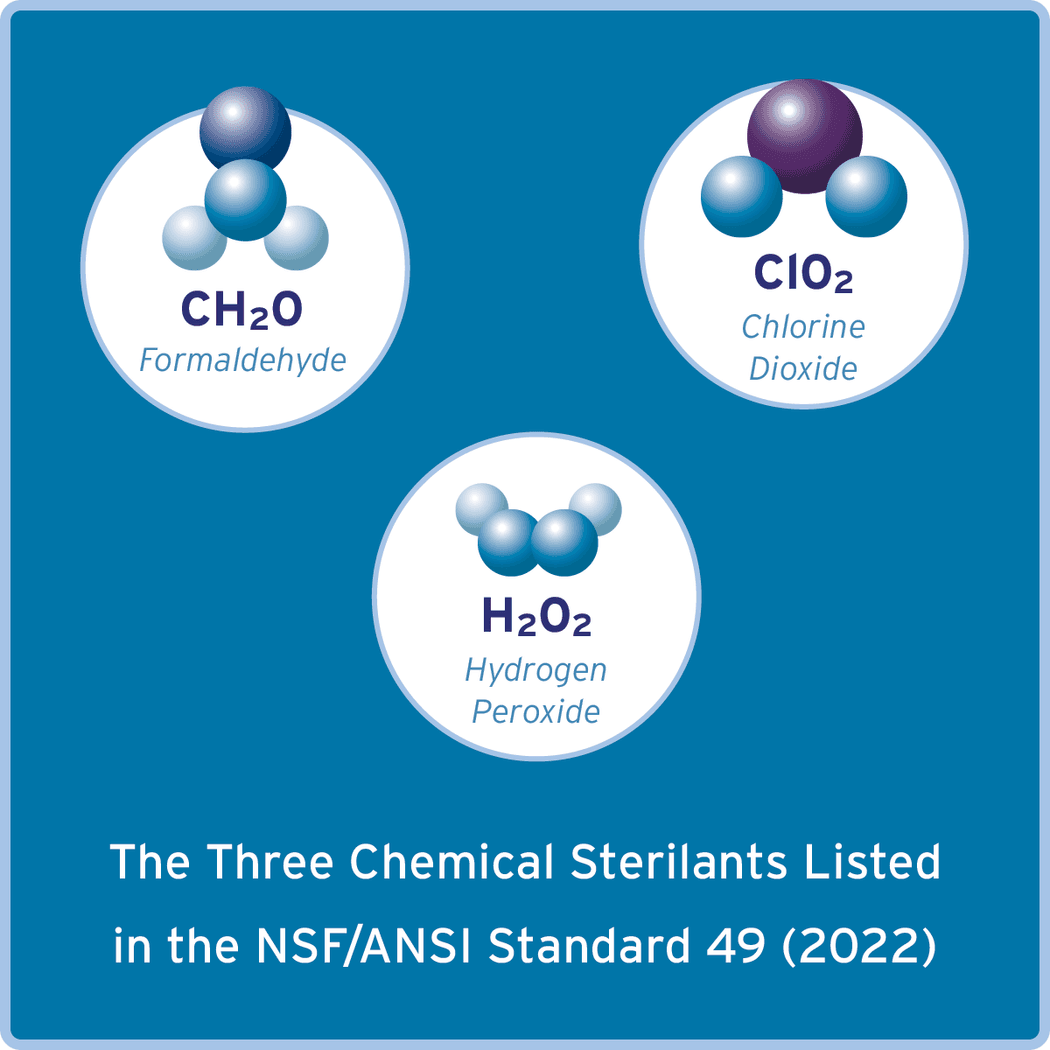
Credit: NuAire
Specific considerations may include:
1. What hazardous materials (biological, chemical, radiological, oil, heavy metals, etc.) have been used in the BSC
2. Whether hazardous materials have been spilled in the BSC
3. The characteristics of the space and equipment to be decontaminated
4. The chemical’s material compatibility
5. The safety of the chemical sterilant
6. The total time needed for gas decontamination
7. The ability of the chemical to penetrate substances and distribute in the BSC
8. The recommendation or approval (NSF, EPA, etc.) of the chemical
9. Whether the chemical leaves residues on surfaces that must subsequently be neutralized or cleaned3,7
There are advantages and disadvantages to each chemical and the specific method of application, which will be described further below. However, for all of these gas decontamination methods, it is critical to note that they should only be performed by an individual with the appropriate experience who has been trained on the specific, validated methodology. They must also have the necessary monitoring equipment and personal protective equipment to safely administer and supervise the gas decontamination treatment.3-6 These chemicals, especially at the concentrations used in gas decontamination, are very effective at killing viable biological materials which is why they are used for these processes. Correspondingly, they can be toxic or otherwise hazardous to human health when used, and formaldehyde is a known carcinogen.3,5-7
Formaldehyde
USE: Decontamination with formaldehyde gas can be performed by heating paraformaldehyde or boiling formalin.6 This method has been used to decontaminate BSCs and laboratory spaces for many years3,8 and a protocol exists in the NSF/ANSI Standard 49 (2022)5 for BSC gas decontamination with paraformaldehyde.
TEMPERATURE and HUMIDITY: The BSC must be at 70ºF (21ºC) or higher and the humidity should be between 60 and 85 percent for formaldehyde treatment.3,5,6,9
SAFETY: Formaldehyde is a known human carcinogen3,6,7 so it is critical that the BSC and room are well ventilated prior to re-entry without respiratory protection.6 The gas produces an odor that can be smelled.7,9
PENETRATION: Formaldehyde is a true gas with good penetration of all areas of the BSC.3,6,7 A previous study of gaseous formaldehyde decontamination noted some incomplete killing of biological indicators (Geobacillus stearothermophilus spores used to test the effectiveness of chemical sterilization) during BSC treatment. The authors suspected that the HEPA filters were absorbing some of the formaldehyde and the presence of water was limiting the effectiveness of the gas decontamination.8 This may have been due to the fact that HEPA filter frames were previously made of particle board wood, which are no longer used, as the industry has switched to aluminum frames. However, an NSF validated procedure for BSC gas decontamination using paraformaldehyde exists that should overcome these described penetration limitations including “bumping” on the BSC blower and/or using fans to promote circulation.5,7
CYCLE TIME: The contact time for formaldehyde gas decontamination is relatively long, with the BSC needing to sit for at least six hours or a preferred 12 hours of treatment.5,7,9 This results in the entire BSC gas decontamination cycle taking at least nine to 15 hours.7
MATERIAL COMPATIBILITY: Formaldehyde has been noted to have the best material compatibility of all of the gas decontamination methods.7
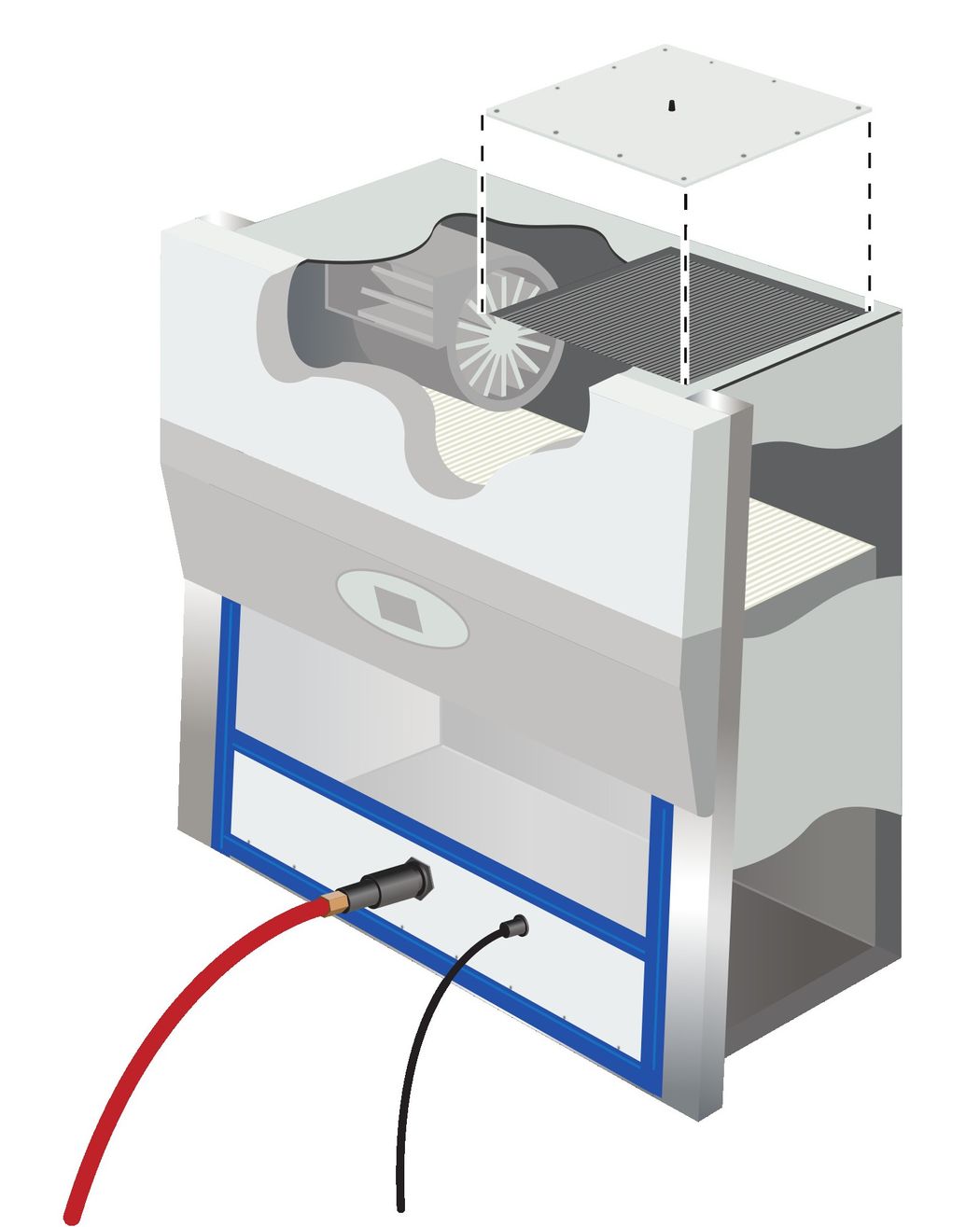
All openings must be sealed to ensure adequate chemical circulation within the device and to prevent any leakage during gas decontamination.
Credit: NuAire
NEUTRALIZATION: This gas must be fully neutralized with ammonia, usually in the form of ammonium carbonate, before it is safe to remove the formaldehyde from the BSC.5,6
RESIDUAL: Formaldehyde leaves behind a chemical residue that must be wiped down, often with ammonia, after use.6,7 This residue can be toxic7, a chemical irritant7, and impact future work with biological materials in the BSC if it is not adequately removed.9 We try to minimize any critical parts within the contaminated area of a biosafety cabinet. To minimize any chance of having to go in and replace that part, because everything has a failure potential in it.
Chlorine dioxide
USE: Decontamination with chlorine dioxide gas has been performed in laboratory rooms, equipment, glove boxes, other BSCs, and incubators.3 Two protocols exist in the NSF/ANSI Standard 49 (2022)5 for BSC gas decontamination with chlorine dioxide gas. The first is using a fixed amount of gas based on the BSC size, similar to the formaldehyde method.5,7,9 The second is using a fixed concentration of chlorine dioxide gas.5,7,9
TEMPERATURE and HUMIDITY: The BSC must be at 60ºF (15ºC) or higher and the humidity should be between 60 and 85 percent for chlorine dioxide treatment.3,5
SAFETY: Chlorine dioxide is not a known human carcinogen6,7,9 and the gas produces an odor that the user can smell.7 Unlike sodium hypochlorite, trihalomethanes are not a byproduct of chlorine dioxide and there is no noted reaction to ammonia products.3
PENETRATION: Like formaldehyde, chlorine dioxide is a true gas7,9 that is easily able to penetrate and distribute through rooms, HEPA filters, and ducts.10 Circulation of gaseous chlorine dioxide during BSC decontamination using the BSC or an external blower is still recommended.5
CYCLE TIME: The contact time for chlorine dioxide gas decontamination is fairly short, with the BSC needing to sit for between 45 and 85 minutes depending on the method and concentration used.5 This results in the entire BSC gas decontamination cycle taking between three to four hours.7 Chlorine dioxide gas can be generated on demand3, and the decontamination process has been described as being easier to control than formaldehyde.6 The gas decontamination process can be impacted by the presence of light in the space(s) being treated.3
MATERIAL COMPATIBILITY: This chemical sterilant is an oxidizer/selective oxidant7,9, however, BSC gas decontamination may be performed infrequently enough that no adverse material reactions may be observed.7,10 Validation testing for gas decontamination subjected the BSC to at least 10 chlorine dioxide cycles, which is proposed to be more than should be expected for the lifetime of a BSC, and no material impacts were seen.9 Use of chlorine dioxide in the field for over five years and 100 decontamination cycles was described with no noted corrosion of the BSC.10 Chlorine dioxide gas has been noted as being compatible with stainless steel, certain plastics (polyethylene and polypropylene), anodized aluminum, nylon, and polytetrafluoroethylene chemical coating.9
NEUTRALIZATION: Chlorine dioxide gas does not need to be neutralized7, but a scrubber should be used to remove the gas from the BSC.5,9
RESIDUAL: No residuals that needed to be wiped up after treatment have been noted with chlorine dioxide gas treatment.7,9 The biosafety cabinet’s front access panel must be sealed to ensure adequate chemical circulation within the device and to prevent any leakage during gas decontamination.
Hydrogen peroxide
USE: Decontamination with hydrogen peroxide vapor has been performed in laboratory rooms, facilities, isolators, gloveboxes, and other BSCs.3,7,12 Two protocols exist in the NSF/ANSI Standard 49 (2022)5 for BSC gas decontamination with hydrogen peroxide vapor, however, they rely on the manufacturer’s and other regulations to set the exact cycle parameters. Depending upon the manufacturer’s protocol, either a fixed amount of vapor based on the BSC size, or sensor-based concentration value is used, but they differ in the location of the chemical generator (inside or outside of the BSC).5
TEMPERATURE and HUMIDITY: The BSC must be between 60 and 90ºF (15 and 32ºC) or higher and the humidity should be between 10 and 85 percent for hydrogen peroxide treatment.3,5
SAFETY: Hydrogen peroxide is not a known human carcinogen6,7, but is listed as an ACGIH A3 animal carcinogen.7 The vapor does not generate an odor.7
PENETRATION: Unlike formaldehyde and chlorine dioxide, hydrogen peroxide is produced in vapor phase and is not a gas.7 This vapor has been noted to have limited distribution and penetration.10 Circulation of hydrogen peroxide during BSC decontamination using the BSC or an external blower is recommended.5
CYCLE TIME: The contact time for vaporized hydrogen peroxide decontamination is fairly short, with some studies noting the BSC needing to sit for around 60 minutes to be sporicidal3, however, the actual cycle time and concentration will vary by manufacturer and equipment.5 This results in the entire BSC decontamination cycle taking between four to seven hours.7 Hydrogen peroxide vapor can be generated on demand5 and can be performed as either a wet or dry process.7 The decontamination process has been described as being easier to control than formaldehyde.6
MATERIAL COMPATIBILITY: Hydrogen peroxide is an oxidizer, however, BSC decontamination may be performed infrequently enough that no adverse material reactions may be observed.7 Some users note condensation of the vapor that can damage paint, surfaces, and electronics.10
NEUTRALIZATION: Hydrogen peroxide vapor does not need to be neutralized as the end products are non-toxic (water and oxygen)3, but an aeration cycle should be used to remove the vapor from the BSC.5
RESIDUAL: No residuals that need to be wiped up after treatment have been described for use of vaporized hydrogen peroxide.7
Cost considerations for gaseous decontamination
Based on the chemical sterilant selected and the method of gas decontamination performed, the cost of a BSC treatment may vary.13 Often formaldehyde treatment is the least costly because of the lower price of the chemical sterilant itself and the use of heating devices such as an electric frying pan to generate formaldehyde gas instead of a more complicated generation unit. Vaporized hydrogen peroxide can be the most expensive option when using a generation unit as opposed to an electric frying pan. The price of the chemical sterilant may vary depending on the actual material and quantity purchased.13
The location of the biosafety cabinet and the duration of the gas decontamination cycle also impact the cost.13 The BSC gas decontamination technician will need to disconnect the BSC from the building exhaust if it is hard ducted or canopy connected to allow for sealing the cabinet. This can add to the total amount of time that individual needs to be on site. Additionally, the technician will need to seal the BSC, the time for which can vary by BSC type, and set up the safety area around the BSC before beginning the decontamination. If the BSC is in a high traffic area and cannot be safely gas decontaminated during the working day, the company may charge more for doing the gas decontamination after standard work hours.13 If multiple BSCs need to be gas decontaminated at one time, the number that can be treated in one day may be limited by the number of gas or vapor generator units that can be brought and used in one day at one location.13
How to prepare for gas decontamination of your biosafety cabinet
When asked about what the user should do to prepare for BSC gas decontamination, Mike Regits, President of DRS Laboratories Inc., said that the BSC user should be involved in “coordination of the whole process. They should know everything or be aware of when that person is coming in to decontaminate that [BSC] from the time they’re walking in until the time they leave and receive a report.”13
Certain preparations of your biosafety cabinet itself, your HVAC system connections, and the area surrounding the BSC are necessary to ensure proper and safe gas decontamination. Regits says that the user should know that the technician will “need some kind of work area to bring their equipment in and set up their equipment up. That person needs space around that [BSC] to provide that service, to provide that process.”13
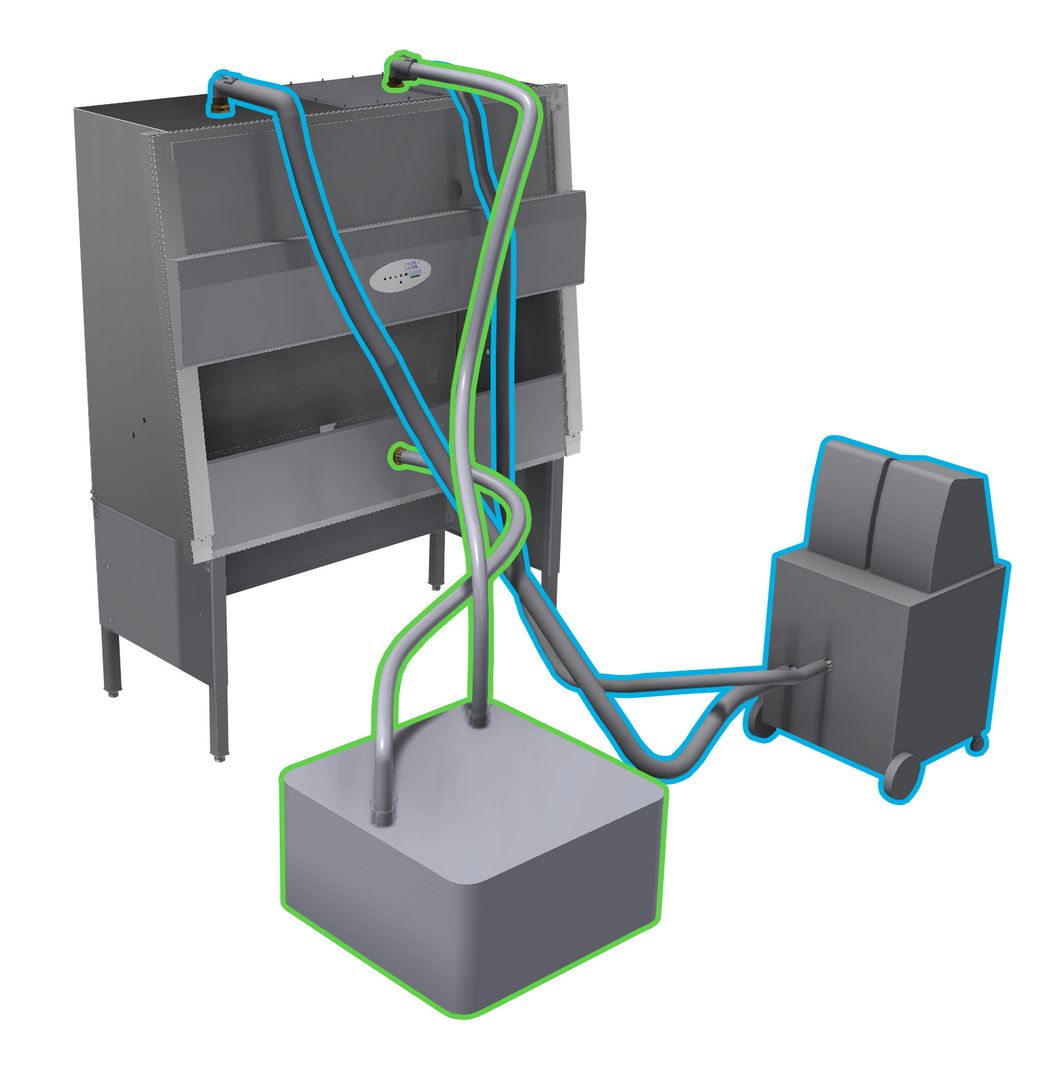
A sealed BSC with possible connections to two different vaporized hydrogen peroxide generation units. Option 1 (outlined in blue): H2O2 vapor generator feeding into top of biosafety cabinet. Option 2 (outlined in green) H2O2 vapor generator feeding into front of biosafety cabinet.
Credit: NuAire
Depending on the type of connection your BSC has, if any, to the building’s exhaust system, the process for isolating the BSC from the exhaust system will be different.5 Generally, the BSC should not allow the chemical sterilant to enter the building exhaust system5 or recirculate back into the laboratory6 during the gas decontamination treatment process. The BSC must be sealed to maintain the chemical sterilant inside the device3-7 and a method should be available to monitor for leakage throughout the gas decontamination process.4,5,7 A safe area around the BSC should be vacated and appropriate signage should be posted based on the chemical in use that notes the time the space is unavailable for use and emergency contact information of the treatment provider.5 The laboratory user “should know when the service provider can come in to do the decon, who’s going to be in the area [for] the notifications” according to Regits.13 Negative pressurization of the space surrounding the BSC should be considered to protect laboratory staff and the environment.5
The use of biological indicators to ensure sterilization (killing of at least 106 bacterial spores) is not strictly required for sterilants for which NSF has a pre-validated gas decontamination procedure.7
How to prepare for gas decontamination of your biosafety cabinet
However, depending on the chemical used and the reason for performing the gas decontamination it may be prudent to use biological indicators or chemical indicators and wait for their results prior to maintaining, moving, or disposing of the BSC. During the gas decontamination cycle, the gas or vapor must be circulated throughout the BSC, which can be achieved using either the BSC’s internal blower or an external source.5,9,12 This external chemical circulation method may be especially important if the BSC blower is not functional5, which has been noted as a common reason to perform a gas decontamination cycle.11
After the gas decontamination contact time, neutralization, scrubbing, and/or aeration must take place to reduce the level of chemical sterilant to a safe level before the area can be released to the user.5 Monitoring equipment should be used to check for the presence of the chemical sterilant, especially at levels hazardous to human health, in the area.5 Once the treatment is completed and results are received from any biological indicators or chemical indicators that were used, the lab user should be provided a report by the company performing the treatment. This report should detail at least the gas decontamination methodology used, the sterilant’s EPA registration number, specifics on the BSC that was treated, the results of any testing, and when the treatment was performed.13
How to find a qualified gas decontamination provider
Often the company that is going to certify your BSC is the same as the one that will conduct the gas decontamination of your BSC.13 Since gas decontamination is a part of the NSF/ANSI 49 standard’s informative annex 25, NSF’s list of accredited biosafety cabinet field certifiers (Enhanced Accreditation Program) is a good place to begin your search for a company that can perform this service. Additionally, you can contact the manufacturer of your BSC to see if they have a recommended company in your area, including those that have been trained to work with their specific BSCs.13 If you know what chemical sterilant you plan to use for your BSC gas decontamination in advance, you can also contact the manufacturer of a decontamination system for recommendations.13
Once you have selected a company to perform the gas decontamination you need to ensure that they are qualified and can conduct the treatment safely.13 Consider asking them for their risk assessment form to determine what chemical treatment method is applicable for your needs and a sample report from a completed gas decontamination.13 Inquire about their methodology for gas decontamination and whether they are using an EPA registered chemical sterilant13 as described in the NSF/ANSI Standard 49.5 And finally, ask about their safety procedures for performing the gas decontamination, monitoring the area during treatment, releasing the area back to you as safe to enter, and whether their technicians have appropriate respiratory protection medical clearance and fit testing, as appropriate, to wear their respirators during treatment.13
Biosafety cabinet design for ease of decontamination
“We try to minimize any critical parts within the contaminated area of a biosafety cabinet. To minimize any chance of having to go in and replace that part, because everything has a failure potential in it” says Bill Peters, President and CEO of NuAire, Inc.14 However, there are a few design features included in or available for biosafety cabinets that may facilitate gas decontamination. Based on the NSF/ANSI 49 standard, the BSC must be able to be decontaminated without being moved5 so consideration for building HVAC connection, including gas-tight dampers, should be made prior to needing to gas decontaminate the BSC. Any gas-tight valves, dampers, supply, return, or recirculation ports should be provided on the clean side components of the BSC including filters and any apertures for the supply or exhaust.5
The BSC should also have front access and exhaust openings that can be easily sealed and made gas-tight.5 When asked about sealing BSCs, Peters noted “Flatness is the best thing. No question.”14 If the BSC blower motor is functional during gas decontamination it can be used to help circulate and distribute the chemical sterilant.5 This can be accomplished using a pre-programmed gas decontamination BSC cycle that includes the appropriate recirculation frequency, timing, and duration depending on the chemical sterilant’s validated procedure. However, overall, there are not many design aspects noted that strongly impact the BSC gas decontamination treatment. “From a design point of view, it’s going to be front accessibility, ease of disassembly, those kind of features with smooth surfaces for sealing” says Peters “The rest of it is on the process side of the world.”14
Conclusion
Depending on your laboratory’s biological materials handling and processes, gas decontamination of your BSCs will likely be a very infrequent service, however, it is important to understand when it may be advantageous or required to have this treatment done. The EPA registered chemical sterilants used to perform gas decontaminations of BSCs all have advantages and disadvantages including safety, penetration, cycle time, material compatibility, and cost. The more you know about the process and your BSC’s design features, the better you will be able to prepare for and select a qualified BSC gas decontamination provider.
References:
1. Baron JL. (2023). "Biosafety Cabinet Surface Decontamination Considerations." https://www.nuaire.com/resources/surface-decontamination-in-a-biosafety-cabinet
2. Controlled Environment Testing Association (CETA). (2020). "CETA Application Guide CAG-004: Biological Decontamination and Disinfection of Accessible Surfaces in Biosafety Cabinets."
3. CDC/NIH. "Biosafety in Microbiological and Biomedical Laboratories (BMBL) 6th Edition." https://www.cdc.gov/labs/BMBL.html
4. World Health Organization. "WHO Laboratory Biosafety Manual: Biological Safety Cabinets and Other Primary Containment Devices" monograph: https://www.who.int/publications/i/item/9789240011335
5. NSF/ANSI 49 – "2022 Biosafety Cabinetry: Design, Construction, Performance, and Field Certification Informative Annex 2" (formerly Annex G): pp 143-159.
6. World Health Organization. "WHO Laboratory Biosafety Manual: Decontamination and Waste Management" monograph: https://www.who.int/publications/i/item/9789240011359
7. Czarneski MA, and K Lorcheim. (2011). "A Discussion of Biological Safety Cabinet Decontamination Methods: Formaldehyde, Chlorine Dioxide, and Vapor Phase Hydrogen Peroxide." Applied Biosafety 16 (1): 26-33.
8. Abraham G, Le Blanc Smith PM, and S Nguyen. (1997). "The effectiveness of gaseous formaldehyde decontamination assessed by biological monitoring." Journal of the American Biological Safety Association 2 (1): 30-38.
9. Luftman HS, Regits MA, Lorcheim P, Lorcheim K, and D Paznek. (2008). "Validation Study for the Use of Chlorine Dioxide Gas as a Decontaminant for Biological Safety Cabinets." Applied Biosafety 13 (4): 199-212.
10. Girouard DJ and MA Czarneski. (2016). "Room, Suite Scale, Class III Biological Safety Cabinet, and Sensitive Equipment Decontamination and Validation Using Gaseous Chlorine Dioxide." Applied Biosafety 21 (1): 34-44.
11. Fey G, Klassen S, Theriault S, and J Krishnan. (2010). "Decontamination of a Worst-case Scenario Class II Biosafety Cabinet Using Vaporous Hydrogen Peroxide." Applied Biosafety 15 (3): 142-150.
12. Frey G, Robertson C, and J Krishnan. (2020). "Decontamination Validation of a Class II Type A2 Biosafety Cabinet during Laboratory Fumigation." Applied Biosafety 25 (1): 48-52.
13. Michael (Mike) Regits, President, DRS Laboratories Inc. Personal communication.
14. William (Bill) Peters, President/CEO, NuAire Inc. Personal communication.
Contributors & Special Thanks to:
Julianne L. Baron, president of Science and Safety Consulting, provides biosafety and biorisk guidance and training to facilitate safe and secure biological research and to prepare organizations for infectious diseases and pandemics. Science and Safety Consulting also facilitates successful scientific communication for technical and non-technical audiences. Connect with an expert: www.scienceandsafetyconsulting.com
Michael Regits, president of DRS Laboratories, Inc., has over 35 years of experience working with decontamination technologies, and is the creator of the Mini-Chlorine Dioxide System (MCS), a patented apparatus for biosafety cabinet decontamination that uses chemicals that he has registered with the EPA for this exact purpose. An expert in the field, Mike is also a key contributor to the NSF/ANSI 49 standard’s informative annex on gaseous decontamination of BSCs. To learn more: https://drslaboratories.com/
Bill Peters, president/CEO of NuAire, Inc., and has over 35 years of experience engineering primary barriers and engineering controls for life science research, pharmacy compounding, animal research and other industries. A member of the NSF committee for biosafety cabinetry, he has also contributed to articles for the Controlled Environment Testing Association (CETA).
Learn more at: www.nuaire.com














