Core laboratories are essential to the scientific research community at the departmental, campus, and, in many cases, regional level. Cores provide access to sophisticated tools and resources that individual principal investigators might find financially out of reach.
In many cases, the decision to start or fund a new core lab is based on securing funding for a new piece of equipment or research process, and often precedes selection of suitable space, sometimes leaving owners scrambling to seek out compatible space in existing buildings. This article explores how to transform aging, underused spaces into specialized core labs. Through a case study detailed by architecture firm CO Architects—the cGMP facility at the University of Southern California (USC)—we will illustrate the process of revitalizing underutilized, aging spaces into high-performance, high-value research hubs.
Assessing the potential in underused spaces
Repurposing an underused space starts with a rigorous evaluation of its architectural, structural, and mechanical fitness. This involves:
1. Evaluation process—Conduct a thorough assessment of the existing space to understand its capabilities and limitations. This includes examining structural fitness and floor stiffness for vibration-sensitive equipment, inspecting rooftop space for potential new mechanical equipment, evaluating the supply and exhaust air capacity of existing HVAC systems, and assessing existing power.
2. Codes and standards—Identify the relevant building codes, applicable National Fire Protection Association standards, institutional environmental health and safety policies, campus laboratory standards, and best practices for specialized containment and clean spaces to detect if there are any obstacles to achieving compliance in the existing space from the outset. If drawings are available, review wall and door fire ratings and existing control areas to understand how the renovation will impact or be impacted by code.
3. Equipment planning—Anticipate the challenges of accommodating large and sophisticated equipment with multiple infrastructure demands. Early engagement with the equipment vendor is critical to understanding site requirements, such as the delivery pathway, freight elevator capacity, minimum room dimensions, overhead clearances required for the equipment, and many other details.

USC cGMP lab “Before”: The existing storage area provided welcome overhead clearances, but diagonal braces challenged the layout.
Credit: CO Architects
Essential questions before design
Before embarking on the design process, it’s crucial to address several key questions:
- Special criteria—What are the special criteria of this lab and/or the equipment in the lab? Core labs must often be designed to accommodate equipment that can both produce and be sensitive to factors of vibration, electromagnetic interference (EMI), temperature, humidity, and high air changes. Early collaboration with equipment manufacturers and special consultants (e.g. vibration or EMI) is strongly recommended to determine the feasibility of the proposed site from physical and cost perspectives. An early “deep dive” into these special criteria can quickly identify whether expensive specialized mechanical, electrical, and plumbing (MEP) systems or instrument shielding are required, and can often be the determining factors as to whether the proposed site is suitable for the core lab.
- System capacity—Can the current HVAC, electrical, and plumbing systems handle the loads of the core lab? For example, will a new local air handling unit (AHU) and dedicated ductwork be required? Some facilities groups can evaluate this in-house, or a third party can be brought in to verify capacity.
- Airflow management—How will the exhaust air be routed through an occupied existing building? Ensuring proper ventilation and exhaust pathways is critical.
- Building occupants—Who are the other principal investigators or tenants in the building? What other major scientific equipment is in the building? Understanding the building’s current usage helps in planning the lab’s integration into ongoing research activities.
- Service space—How much dedicated service space will be needed to serve lab equipment? This includes space for maintenance access and equipment servicing, such as cylinder delivery.
- Compatibility—What specialized equipment are the current tenants using? Coordinate with the owner to survey existing research and building equipment that may be incompatible with the equipment or activities in the proposed core lab.
- Code compliance—What will we need to bring up to code? Ensuring the space meets all regulatory requirements is essential.
Case study: USC Current Good Manufacturing Practice (cGMP) facility
The USC/CHLA Cell Therapy Program (CTP) was initiated in 2021 with investments from the Keck School of Medicine (KSOM) of USC, Keck Medicine (KM) of USC, and Children’s Hospital Los Angeles (CHLA). The primary goal of the program was to promote clinical translation of a growing body of preclinical work in cell and gene therapy at each institution and maximize success of the entire translational paradigm. By generating a mature translational ecosystem with state-of-the-art laboratories, hands-on support, and educational offerings, the USC CTP cGMP lab provides researchers with the right core lab and proficient guidance to successfully translate early-stage ideas into clinically viable products in a robust and cost-effective manner.
Ultimately, CTP offers researchers/collaborators a meaningful partnership and a wide range of services, from strategic planning to product manufacturing and quality control testing. By aligning the scientific and clinical objectives of the project to Good Manufacturing Practices (GMP) and other relevant regulatory requirements, they develop and optimize vein-to-vein workflows and identify improvement opportunities in the process. They also partner with external stakeholders to provide a one-stop-shop solution that utilizes some of the most advanced analytical and proof-of-principle approaches in the sector.

The samples processing room serves all laboratory activities.
Credit: Tom Bonner
With the final facility design completed during the COVID-19 pandemic, initial construction took place in early 2021. Within a year, the facility was fully constructed and commissioned. With new research projects and clinical partners’ demands lining up, the facility became operational in 2023.
The project began with a 10-week feasibility study to evaluate the space and its potential in the portion of an unused basement in a 1990s-era biosciences lab tower. The feasibility study evaluated existing architectural, structural, and mechanical systems. Having a high floor-to-floor height for the density of overhead ductwork that would serve the lab was critical in the selection of the space. Once the feasibility study concluded that the build-out of a cGMP lab was possible, USC moved forward with an NIH Grant Application to try to secure additional funds. A two-month NIH grant application process was undertaken to document and describe the facilities portion of the future cGMP lab, integrating facilities drawings with the scientific narrative. Although the grant pursuit was not successful, planning for the facility proceeded onward. The total design and construction phase lasted 24 months, involving extensive user meetings with USC as well as initial advising by stem cell facility expert Gerhard Bauer, PhD; later and final advising was conducted with the future facility director, Dr. Mohamed Abou-el-Enein, MD, PhD.
To stay on schedule and on budget—and meet stringent cGMP environmental criteria—a modular cleanroom package was designed using a design-assist approach, with the panel manufacturer participating in the design process. Modular panels were integrated with existing walls and structures in the basement area. Modular cleanroom panels were mocked up on-site and prefabricated for installation. While these panels solved certain problems, they also presented new challenges; since they were fabricated overseas, COVID-19 supply chain bottlenecks had an impact on their delivery schedule.
One of the most challenging aspects of the project was finding space for the new dedicated AHU, which could have required 50 percent of the area devoted to the lab itself. The architects took advantage of the high floor-to-floor height to stack the twin AHUs that were required to meet the owner’s requirement for 100 percent redundancy in the system. A concurrent effort was made to find pathways for exhaust air, outside air, makeup air, and supply air that didn’t cause major disruptions to existing research laboratories and building operations, as the existing shafts were at capacity. The team was able to route major ductwork through an existing vestibule shaft, and through a copy room and break room that repeated on each floor, which had an impact on counter space at these areas.
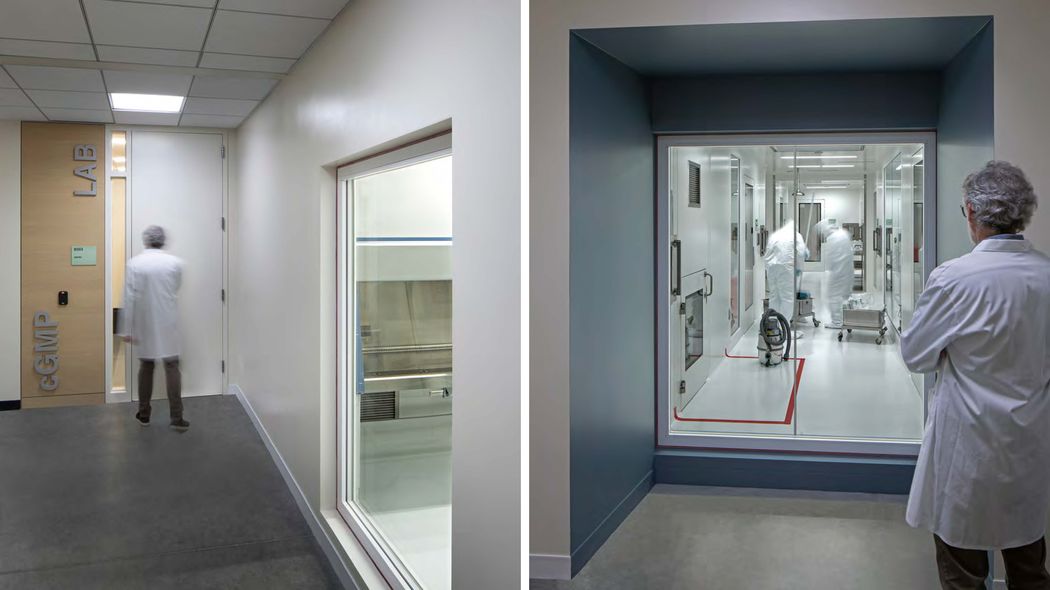
The design team provided opportunities for visitors to observe operations in this clean facility from the public corridor.
Credit: Tom Bonner
Wrapping up: The benefits of repurposing aging spaces
Repurposing underused spaces into specialized core labs offers significant benefits:
1. Cost effectiveness—Renovations are often more cost effective than new constructions, as they utilize existing infrastructure, but thorough pre-work is necessary to realize potential benefits.
2. Maximized utilization—Transforming underused spaces enhances the overall value and utility of the institution’s real estate.
3. Increased research funding—Universities attract funding and top-tier talent by expanding research capabilities.
Transforming aging and underused spaces into highly specialized core labs is a feasible and beneficial endeavor. By leveraging strategic planning, stakeholder engagement, and meticulous project management, institutions can turn underwhelming spaces into thriving centers of scientific innovation.












