Measuring cell characteristics: why do we do it? Cells constitute discrete units of biological function and serve as starting points in a myriad of studies to identify and map many of the basic biochemical and physical processes of life. Of the ways to analyze cells en masse, flow cytometry is a widely used and respected method to get highly specific information about individual cells.
Flow cytometry is a cell analysis technique first used in the 1950s to measure the volume of cells in a rapidly flowing fluid stream as they passed in front of a viewing aperture. Since then, innovations have culminated in the modern flow cytometer, which is able to make measurements of cells in solution as they pass by the instrument’s laser at rates of 10,000 cells per second (or more). Today’s instruments offer an increased number of detectable fluorescent parameters (from one or two up to 30 or more), all measured at the same time on the same cell. Because of its speed and ability to scrutinize at the single-cell level, flow cytometry offers the statistical power to rapidly analyze and characterize millions of cells.
There are numerous potential applications of analysis using flow cytometry, including the detection and measurement of:
• Protein expression—throughout the entire cell, even the nucleus
• Protein post translational modifications—includes cleaved and phosphorylated proteins
• RNA—including IncRNA, miRNA, and mRNA transcripts
• Cell health status—from viability to late-stage apoptosis or programmed cell death
• Cell cycle status—providing a powerful tool to assess cells in G0/G1 phase versus S phase, G2, or polyploidy, including analysis of cell proliferation and activation
• Identification and characterization of distinct subsets of cells within a heterogeneous sample—including distinguishing central effector memory cells from exhausted T cells or even regulatory T cells
• Semen sorting of the X and Y chromosomes based on the weights of the X or Y bearing sperm
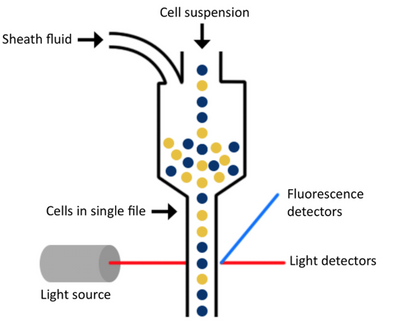
There are three main components of a flow cytometer that allow all of this to be possible. They are the fluidics, optics, and electronics.
The fluidics system of a flow cytometer is responsible for transporting sample from the sample tube to the flow cell. Once through the flow cell (and past the laser), the sample is either sorted (in the case of cell sorters) or transported to waste.
The components of the optical system include excitation light sources, lenses, and filters used to collect and move light around the instrument and the detection system that generates the photocurrent.
The electronics are the brains of the flow cytometer. Here, the photocurrent from the detector is digitized and processed to be saved for subsequent analysis.
There are a lot of important things to consider when it comes to flow cytometry; one being auto-fluorescence (AF). Products have
inherent background fluorescence and the trick is to find markers with different colors and excitation wavelengths that allow the markers to be pulled out. Another thing to consider is debris, as debris doesn’t stain with the dead cell marker. This results in all debris being counted as live cells, underestimating presence of cell markers and overestimating cell viability. Lastly, there are tandem-dyes that provide much higher wavelength fluorescence emission. These have the ability to confirm presence of dual markers on one sample for increased specificity (e.g. CD90+ and CD45-) or complicated DoE to make sure markers all play together nicely. Therefore, it won’t bind to debris and are out of the range of the AF.
One of the most important things to consider is the sheath fluid used. Once the sample is placed on the flow cytometer, the sample is taken up into the instrument, and the cells are surrounded by a physiological buffer called sheath fluid. The fluidics system—the tubing, pumps, and valves—organizes the initial sample suspension into a single-file stream of cells as they make their journey through the flow cytometer for analysis. Because of the complex hydrodynamic focusing technology of the instrumentation and depending on your experimental needs, choosing the correct sheath fluid solution is an important decision for a flow cytometry core manager or lab manager responsible for the optimal performance of their lab’s instrumentation. It is important that you take things like solubility into account to ensure you are not dissolving what you are attempting to count; that everything in the formulation supports the ability to maintain cell viability while being used; that the components used aren’t going to add endotoxin or microorganisms that could impact the cells as most cytometers are not closed systems; that you are using the proper pH and osmolality to ensure you aren’t lysing the cells; and that the formulation is compatible with your specific piece of equipment. If any of these things aren’t set up correctly, they have the ability to bias or prevent you from being able to obtain the results you are looking for.
Choosing the best sheath fluid for your operation is more than just buying something off the shelf and running it through the machine, since problems may arise when the incorrect sheath fluid is used. By taking these points into consideration and employing some best practices for laboratory safety, quality control, and housekeeping, you should be able to minimize the potential of using a problematic sheath fluid and increase overall laboratory efficiencies.
Sheath fluids are certainly more complicated than they seem. As a critical part of flow cytometry testing, it is vital that you take the time to understand the formulation and quality of the sheath fluid being used and how it will impact your samples, products, and processes.
Asa Yoakam is a business development manager for the Cellular Agriculture industry at Lifecycle Biotechnologies. He focuses on expanding Lifecycle’s partnerships with companies in livestock/companion animal health & diagnostics, animal genetics, and the burgeoning field of clean meat. Asa has spent the last seven years building partner relationships in rapid growth markets and has been with Lifecycle since early 2019.












