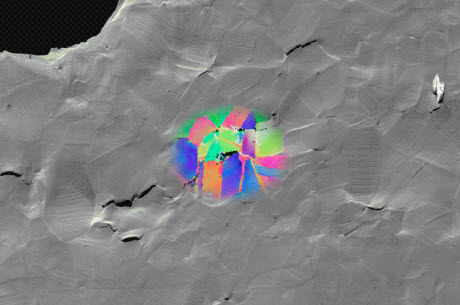
 This scanning electron micrograph shows a sample of metal that fractured due to hydrogen embrittlement. The colored patches show data acquired by electron backscatter diffraction, revealing the grain structure surrounding selected cracks. Such images help show the correlation between microstructure and hydrogen embrittlement, and are used to identify the features that lead to hydrogen-assisted fracture.John Hanson and Matteo SeitaWhen a metal tube lines an oil well thousands of feet below the surface of the ocean, that metal had better be solid and reliable. Unfortunately, the environment in such deep wells is often rich in hydrogen, a gas that can penetrate high-tech alloys and make them brittle — making fractures and leaks more likely.
This scanning electron micrograph shows a sample of metal that fractured due to hydrogen embrittlement. The colored patches show data acquired by electron backscatter diffraction, revealing the grain structure surrounding selected cracks. Such images help show the correlation between microstructure and hydrogen embrittlement, and are used to identify the features that lead to hydrogen-assisted fracture.John Hanson and Matteo SeitaWhen a metal tube lines an oil well thousands of feet below the surface of the ocean, that metal had better be solid and reliable. Unfortunately, the environment in such deep wells is often rich in hydrogen, a gas that can penetrate high-tech alloys and make them brittle — making fractures and leaks more likely.
Now researchers at MIT have figured out exactly which characteristics of a metal structure tend to foster this embrittlement in the presence of hydrogen. More significantly, they have also determined that simple changes in processing can modify the structure in a way that may greatly reduce the chances of damage, extending the safe operating lifetime of such tubing.
Their findings are published this week in the journal Nature Communications, in a paper co-written by MIT faculty members Michael Demkowicz and Silvija Gradecak, postdoc Matteo Seita, and doctoral student John Hanson.
“We want to engineer reliability into the metal,” says Demkowicz, an associate professor of materials science and engineering at MIT. The research was supported by BP, whose well in the Gulf of Mexico was responsible for the 2010 Deepwater Horizon oil spill. (Metal failure was not implicated in that particular spill.)
Resisting failure
Demkowicz says the question he and his team explored was: How do you make materials that are inherently resistant to failure in a harsh environment?
In very deep wells, the environment is often highly acidic. “That means there’s a lot of hydrogen in the environment,” Demkowicz says — conditions that are known to rapidly increase the brittleness of the nickel superalloys used for deep-well linings.
“People have realized for 100 years that metals get brittle when exposed to hydrogen,” Demkowicz says. “In fact, most of the materials problems that dog us today are problems that we have known about for a long time: corrosion, fatigue, many different kinds of failure. But we’re still really bad at predicting them.”
In the case of critical parts, like oil-well casings and reactor coolant lines, that often means that metal parts are replaced early, as a precaution, based on a worst-case analysis of failure. Better predictions, or more resistant materials, could allow components to stay in place longer, resulting in significant savings. The MIT researchers say their work could potentially allow for less-frequent replacement of the nickel superalloy widely used in oil drilling.
Studying the microstructure
“The novelty of our study was the strategy we used for looking at the microstructure of the material, and what is the role of the microstructure in causing embrittlement,” says Seita, one of the paper’s two lead authors. Through this analysis, he and his co-authors found that grain boundaries — the boundaries between individual microscopic crystals — are where cracks tend to get started in a metal. But certain arrangements of these boundaries make it much less likely that a tiny crack will be able to grow larger, they found.
Both the types of grain boundaries and their physical arrangement play an important role in determining how vulnerable a given piece of metal may be to embrittlement and failure, the researchers discovered. The findings were made possible by tests of materials under conditions resembling those that would be faced when in use, Seita says.
Previous investigations had looked at the role of grain boundaries, Hanson says, but only in terms of their overall distribution in the material. “We had to look at individual grain boundaries,” he says, “focusing on individual cracks and single grain boundaries.”
The general thinking had been that certain special grain boundaries are intrinsically resistant to fracture, Seita says. “People thought that introducing more of these would always lead to more resistance,” he explains, “but the story is more complex than that.”
The researchers found that one particular type of boundary, called a coherent twin boundary (CTB), plays a dual role in fracture. CTBs are much less likely to propagate pre-existing cracks through the metal, but they are also the main features responsible for incipient cracks forming in the first place.
This is “interesting work,” says Alan Turnbull, a senior fellow at the National Physical Laboratory in the U.K., who was not involved in this research. He says the team showed that in the formation of cracks, CTBs “encourage initiation, but resist propagation.”
The work was supported by the BP-MIT Materials and Corrosion Center, the National Science Foundation, and the U.S. Department of Energy.













