In the pharmaceutical and life sciences industries, the integrity and reliability of analytical data are the bedrock of quality control, regulatory submissions, and patient safety. For multinational companies and laboratories, navigating a patchwork of regional regulations for method validation can be a logistical and scientific nightmare. This is precisely the challenge addressed by the International Council for Harmonisation (ICH) and its member regulatory bodies, such as the U.S. Food and Drug Administration (FDA).
The ICH provides a harmonized framework that, once adopted by member countries, becomes the global gold standard for analytical method guidelines. This framework is designed to ensure that a method validated in one region is recognized and trusted worldwide, streamlining the path from development to market. This article will explore the critical, interconnected roles of ICH and FDA guidelines, focusing on the recent, modernized approach introduced by ICH Q2(R2) and ICH Q14, and provide a clear roadmap for putting these principles into practice.
The Role of ICH and FDA in Analytical Method Validation
The ICH is a unique collaborative initiative involving regulatory authorities and the pharmaceutical industry from around the world. Its mission is to develop harmonized technical guidelines that promote global consistency in drug development and manufacturing. Among its most influential contributions are the "Q" series of guidelines, which focus on quality.
- ICH Q2(R2): Validation of Analytical Procedures: This core guideline is the global reference for what constitutes a valid analytical procedure. The recent revision, Q2(R2), has modernized the principles from the previous version, Q2(R1), by expanding its scope to include modern technologies and emphasizing a science- and risk-based approach to validation.
- ICH Q14: Analytical Procedure Development: This new guideline complements Q2(R2) by providing a framework for a systematic, risk-based approach to the development of analytical procedures. It introduces concepts like the Analytical Target Profile (ATP), which helps to proactively define the desired performance criteria of a method from the outset.
The FDA, as a key member of ICH, works closely with the council and subsequently adopts and implements these harmonized guidelines. For laboratory professionals in the U.S., this means that complying with ICH standards is not an optional extra; it is a direct path to meeting FDA requirements and is critical for regulatory submissions such as New Drug Applications (NDAs) and Abbreviated New Drug Applications (ANDAs). These guidelines are the essential reference for anyone needing to demonstrate the fitness-for-purpose of an analytical procedure for its intended use.
Core Validation Parameters: Defining a Reliable Method
ICH Q2(R2) outlines a set of fundamental performance characteristics that must be evaluated to demonstrate that a method is fit for its purpose. While the exact parameters to be tested depend on the type of method (e.g., quantitative assay vs. identification test), the core concepts are universal to analytical method guidelines.
- Accuracy: The closeness of the test results to the true value. It is typically assessed by analyzing a standard of a known concentration or by spiking a placebo with a known amount of analyte.
- Precision: The degree of agreement among individual test results when the procedure is applied repeatedly to multiple samplings of a homogeneous sample. This includes repeatability (intra-assay precision), intermediate precision (inter-day, inter-analyst), and reproducibility (inter-laboratory).
- Specificity: The ability to assess unequivocally the analyte in the presence of components that may be expected to be present, such as impurities, degradation products, or matrix components.
- Linearity: The ability of the method to elicit test results that are directly proportional to the concentration of the analyte within a given range.
- Range: The interval between the upper and lower concentrations of the analyte for which the method has demonstrated a suitable degree of linearity, accuracy, and precision.
- Limit of Detection (LOD): The lowest amount of analyte in a sample that can be detected but not necessarily quantitated.
- Limit of Quantitation (LOQ): The lowest amount of analyte in a sample that can be determined with acceptable accuracy and precision.
- Robustness: A measure of a method's capacity to remain unaffected by small, deliberate variations in method parameters (e.g., pH, flow rate). This is now a more formalized concept under the new guidelines and is a key part of the development process.
ICH Q2(R2) and Q14: A Modernized Approach
The simultaneous release of ICH Q2(R2) and the new ICH Q14 represents a significant modernization of analytical method guidelines. This is more than just a revision; it is a shift from a prescriptive, "check-the-box" approach to a more scientific, lifecycle-based model.
- From Validation to Lifecycle Management: The new guidelines emphasize that analytical procedure validation is not a one-time event. Instead, it is a continuous process that begins with method development and continues throughout the method's entire lifecycle. This includes managing post-approval changes in a more flexible, science-based way, a concept introduced in ICH Q12.
- The Analytical Target Profile (ATP): Q14 introduces the ATP as a prospective summary of a method's intended purpose and desired performance characteristics. By defining the ATP at the beginning of development, a laboratory can use a risk-based approach to design a fit-for-purpose method and a validation plan that directly addresses its specific needs.
- Enhanced vs. Minimal Approach: The guidelines describe two pathways for method development: the traditional, minimal approach and an enhanced approach. The enhanced approach, while requiring a deeper understanding of the method, allows for more flexibility in post-approval changes by using a risk-based control strategy.
- Inclusion of New Technologies: Q2(R2) has been expanded to explicitly include guidance for modern techniques, such as multivariate analytical procedures and other advanced technologies, ensuring that the guidelines remain relevant in an era of rapid technological advancement.
Putting Guidelines into Practice: A Compliance Roadmap
For laboratory professionals, applying these analytical method guidelines requires a strategic shift. It’s about building quality into a method from the very beginning, rather than trying to validate it at the end.
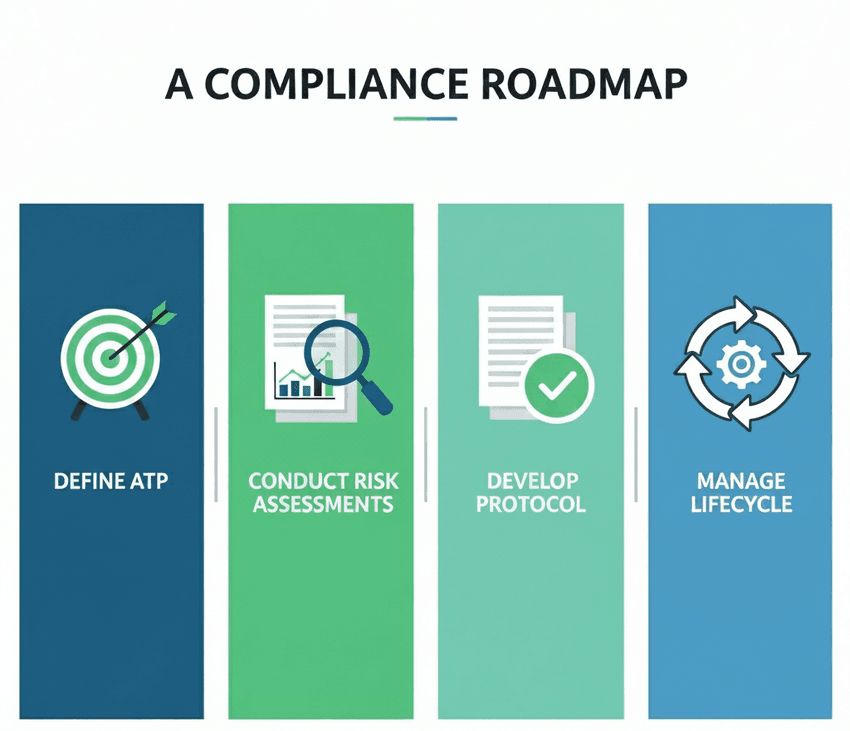
Following a defined process can make analytical method validation easier.
GEMINI (2025)
- 1. Define the Analytical Target Profile (ATP): Before starting development, clearly define the purpose of the method and its required performance characteristics. What is the analyte? What are the expected concentrations? What degree of accuracy and precision is required?
- 2. Conduct Risk Assessments: Use a quality risk management approach (as described in ICH Q9) to identify potential sources of variability during method development. This helps in designing the robustness studies and defining a suitable control strategy.
- 3. Develop a Validation Protocol: Based on the ATP and risk assessment, create a detailed protocol that outlines the validation parameters to be tested, the acceptance criteria, and the experimental design. This protocol serves as the blueprint for your validation study.
- 4. Manage the Method Lifecycle: Once the method is validated and in use, a robust change management system is essential. The new guidelines provide a path for making justified changes to a method without the need for extensive regulatory filings, provided a sound scientific rationale and risk assessment are in place.
The Path Forward: Embracing Modern Analytical Guidelines
The latest analytical method guidelines from ICH and the FDA represent a significant evolution in laboratory practice. They shift the focus from simple compliance to a proactive, science-driven approach to quality assurance. By embracing concepts like the Analytical Target Profile and a continuous lifecycle management model, laboratories can not only meet regulatory requirements but also build more efficient, reliable, and trustworthy analytical procedures. These guidelines empower professionals to stay ahead of the curve, ensuring their methods are not just validated, but truly robust and future-proof.
FAQ: Analytical Method Guidelines
How do ICH and FDA guidelines differ for analytical method validation?
The ICH develops harmonized analytical method guidelines that are globally accepted. The FDA, as a member of ICH, adopts these guidelines for use in the United States. Therefore, for most new drug submissions, following the latest ICH guidelines (like Q2(R2) and Q14) is the key to meeting FDA requirements.
What is the main benefit of the new ICH Q2(R2) and Q14 guidelines?
The main benefit is a shift from a rigid, prescriptive approach to a flexible, science- and risk-based framework. This allows laboratories to better understand their methods, demonstrate quality through a continuous lifecycle approach, and manage changes more efficiently.
Do these guidelines apply to all analytical methods?
The guidelines primarily apply to analytical procedures used for the release and stability testing of drug substances and products for regulatory submission. While they are not mandatory for all methods, their principles are considered industry best practice and can be applied to other analytical procedures following a risk-based approach.
What is an Analytical Target Profile (ATP)?
Introduced in ICH Q14, the ATP is a prospective summary that describes the intended purpose of an analytical procedure and its required performance characteristics. Defining an ATP at the start of method development ensures that the method is designed to be fit-for-purpose from the very beginning.














