The landscape of modern discovery is fundamentally shaped by the capabilities and operational efficiency of the industrial research lab. Within these specialized environments, theoretical science transitions into tangible, scalable technologies, directly impacting product development, manufacturing standards, and regulatory compliance. The success of an industrial research lab hinges not only on the brilliance of its personnel but also on the strategic design of its workflows and the robustness of its governance systems. For laboratory professionals navigating increasingly complex research portfolios, understanding these drivers is critical to ensuring scientific outcomes are reproducible, compliant, and ready for commercial application.
Strategic design and infrastructure for modern industrial research labs
The physical and digital infrastructure of an industrial research lab serves as the foundation for its innovative capacity. Unlike academic settings, the design of an industrial research lab must explicitly prioritize scalability, regulatory adherence, and operational continuity. This necessitates a holistic approach to designing industrial labs that considers not just immediate research needs but also future growth and the integration of advanced technologies.
Effective spatial planning is paramount. Laboratory areas must be modular and flexible to accommodate dynamic research demands, particularly the rapid reconfiguration required for new project starts or shifting technological focuses. Dedicated zones must be established for specialized processes, such as clean rooms for microfabrication, containment facilities for biohazardous materials, or inert atmosphere glove boxes for sensitive chemical synthesis. Crucially, the design must support the segregation of different activities—specifically, the physical separation between exploratory R&D, process development, and final quality control testing—to prevent cross-contamination and maintain data integrity throughout the experimental lifecycle.
Beyond the physical structure, modern laboratories rely heavily on digital infrastructure. The integration of Laboratory Information Management Systems (LIMS), Electronic Laboratory Notebooks (ELN), and sophisticated data analysis platforms is non-negotiable. These systems facilitate audit trails, standardize documentation, and ensure that all experimental data is securely managed and easily accessible, supporting both internal review and external regulatory scrutiny. A well-designed digital architecture allows for seamless data flow, which is a prerequisite for implementing high-throughput screening platforms and other automated methodologies discussed later. The initial investment in resilient, compliant infrastructure prevents costly retrofitting and regulatory issues later in the discovery process.
Key infrastructure considerations:
Component | Functionality Focus | Regulatory Impact |
|---|---|---|
HVAC & Ventilation | Precise temperature, humidity, and pressure control; chemical fume removal. | OSHA/COSHH compliance; sample stability and integrity. |
LIMS/ELN | Centralized data storage, protocol management, audit trails, sample tracking. | FDA 21 CFR Part 11 compliance; IP protection. |
Ergonomics & Safety | Adjustable workstations, clearly marked safety zones, chemical storage management. | OSHA compliance; reduction of operational risk. |
Power & Backup | Stable, conditioned power supply; redundant backup systems for critical equipment. | Operational continuity; prevention of data and sample loss. |
Accelerating the innovation cycle through cross-sector R&D labs and prototyping
In the competitive industrial environment, the speed at which a hypothesis moves from concept to validated prototype is a key differentiator. The modern industrial research lab has evolved into a dynamic hub focused on accelerating prototyping through agile methodologies and interdisciplinary collaboration. This approach is exemplified by the rise of cross-sector R&D labs, which pool expertise and resources across traditionally siloed scientific disciplines (e.g., combining materials science, artificial intelligence, and synthetic biology).
Acceleration is achieved by compressing the traditional linear R&D process into iterative, feedback-driven cycles. Instead of waiting for a final, fully optimized solution, the focus shifts to creating Minimum Viable Prototypes (MVPs) early in the process. This rapid iteration allows for early failure detection, preventing significant investment in dead-end pathways. Laboratory professionals in these environments must be adept at utilizing advanced simulation and modeling tools, reducing the number of physical experiments required and shortening the overall development timeline.
Furthermore, cross-sector R&D labs dismantle organizational barriers that often impede innovation. When physicists, chemists, and software engineers work side-by-side, they bring diverse perspectives to problem-solving, leading to novel solutions that might be missed in traditional discipline-specific labs. For instance, in drug discovery, the integration of automation specialists and data scientists directly into the chemistry lab has drastically reduced the cycle time for compound synthesis and testing. This collaborative model requires specialized training for lab personnel. They must be able to communicate effectively across scientific vocabularies and technological platforms. The flexibility inherent in the design of the industrial research lab must mirror this cognitive flexibility. This enables shared equipment use and streamlined data exchange between groups. This interdisciplinary effort significantly enhances the breadth and quality of the intellectual output.
A crucial component of accelerating prototyping is the use of advanced manufacturing techniques like additive manufacturing (3D printing) for custom tooling, microfluidic devices, and small-batch product components. This capability allows researchers to move from computer-aided design (CAD) to physical testing in hours, rather than weeks, dramatically shortening the loop between design, fabrication, and experimental validation.
Comprehensive risk management in the industrial research lab environment
Effective risk management is integral to the successful operation of an industrial research lab, encompassing safety, regulatory, and financial considerations. For laboratory professionals, risk mitigation is not merely a compliance burden but a strategic activity that ensures the continuity of high-value research and protects the integrity of scientific data.
Operational risk begins with chemical, biological, and physical hazards. A proactive risk management strategy employs a tiered approach, starting with thorough Hazard Analysis and Critical Control Points (HACCP) assessments for every new protocol.
Effective risk mitigation in the industrial research lab requires clear prioritization of control measures:
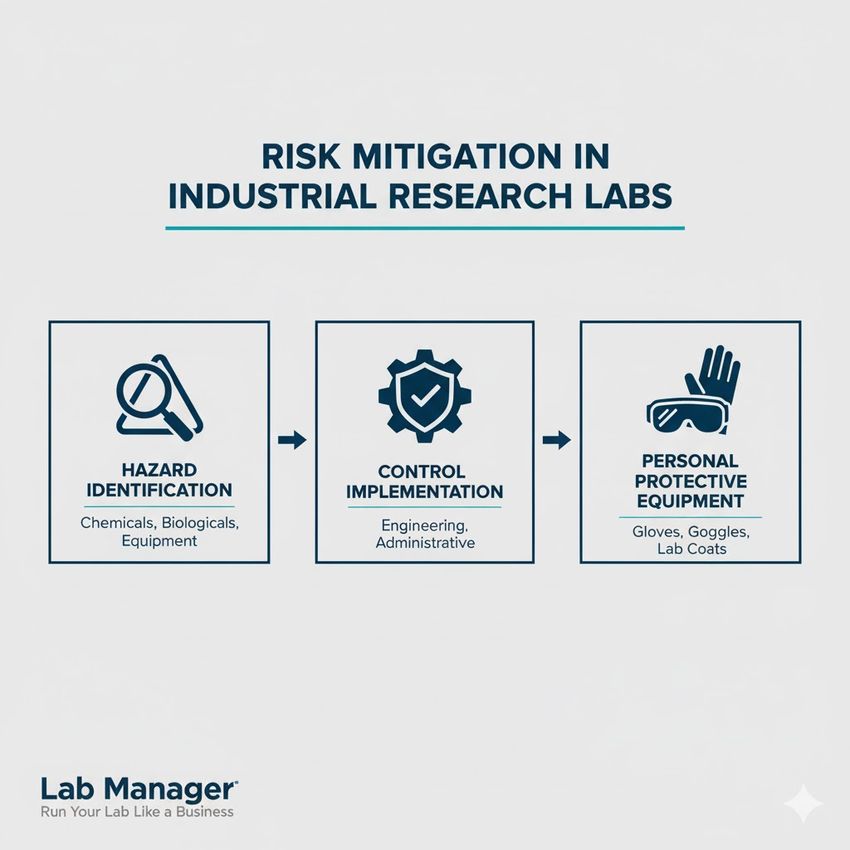
The 3-steps essential for industrial research risk mitigation.
GEMINI (2025)
- Hazard identification: Identifying and quantifying chemical, biological, or physical risks inherent to the protocol.
- Control implementation: Establishing preventive measures, prioritizing robust engineering controls (e.g., fume hoods, contained systems) over administrative controls (e.g., procedural changes).
- Personnel protection: Ensuring all laboratory personnel utilize appropriate Personal Protective Equipment (PPE) tailored to the specific assessed risks.
Standard Operating Procedures (SOPs) must be detailed, accessible, and consistently enforced. They must reflect the guidelines set by authoritative bodies like the Occupational Safety and Health Administration (OSHA) in the United States and similar global agencies.
Regulatory risk presents another critical challenge. Labs operating in regulated industries (e.g., pharmaceutical, medical device, aerospace) must adhere to Good Laboratory Practice (GLP), Good Manufacturing Practice (GMP), and specific governmental regulations. Compliance involves stringent documentation, validated equipment, qualified personnel, and meticulously maintained quality systems. Failures in regulatory compliance can result in product recalls, clinical holds, or complete cessation of operation. This poses a significant financial risk to the organization. Regular, independent audits are essential tools for identifying gaps in the quality system before they lead to non-conformance.
Furthermore, operational continuity must be considered. This includes planning for equipment failure, natural disasters, or external disruptions. The industrial research lab requires robust disaster recovery plans. These plans ensure that irreplaceable samples, reagents, and electronic data are stored securely offsite or redundantly. Regular testing of these recovery protocols verifies the lab's ability to resume critical operations swiftly following an incident, mitigating the financial and temporal risk associated with downtime. This holistic approach elevates risk management from a checklist activity to a core operational standard. (FDA Title 21 Code of Federal Regulations, Part 58, Subpart E - Equipment provides clear requirements for equipment maintenance and calibration, directly impacting risk management.)
Implementing robust QC systems and high-throughput screening platforms
The reliability of a product or process derived from an industrial research lab is directly tied to the integrity of the underlying scientific data. Therefore, the implementation of stringent QC systems is fundamental. Quality control is not an endpoint but a continuous process integrated into every stage of the research workflow, from reagent preparation to final data analysis. Key elements include rigorous instrument qualification, method validation, and adherence to change control protocols.
Method validation is paramount, particularly when utilizing high-throughput screening platforms (HTS). Validation ensures that the analytical method is suitable for its intended purpose. It considers parameters such as accuracy, precision, linearity, range, detection limit, and quantification limit. For HTS, validation must specifically account for the unique challenges of miniaturization and automation. These challenges include potential edge effects, cross-talk between wells, and the consistency of liquid handling robotics. Sophisticated software is employed to monitor these platforms, flagging any deviations from expected performance and providing detailed logs of every action performed.
High-throughput screening platforms have revolutionized discovery by allowing the simultaneous testing of thousands or millions of samples or conditions. This requires specialized QC systems to manage the vast influx of data. Data integrity is maintained through automated checks for plate-to-plate variation, Z'-factor calculation (a measure of assay quality), and the use of reference standards on every plate. The data generated is then automatically processed through validated computational pipelines. This minimizes human error and ensures reproducibility. The transition to automation necessitates a shift in the laboratory professional’s role, focusing expertise on assay development, platform maintenance, and data interpretation, rather than manual execution.
The adoption of international standards is also crucial for QC systems. Compliance with ISO standards (e.g., ISO 17025 for testing and calibration laboratories) provides a globally recognized framework for quality assurance, enhancing the credibility of results generated by the industrial research lab. (International Organization for Standardization (ISO) 17025:2017 specifies the general requirements for the competence, impartiality, and consistent operation of laboratories.)
Strategically managing IP within the innovation pipeline
Intellectual property (IP) is the primary financial asset generated by an industrial research lab. The successful managing IP strategy involves proactive identification, meticulous documentation, and strategic protection of novel discoveries throughout the entire R&D lifecycle. For laboratory professionals, every entry into an ELN and every piece of data collected has potential commercial value. Specific protocols are required to secure that information.
Documentation is the bedrock of IP protection. Every experiment, observation, and result must be recorded in detail, including the date, time, personnel involved, and signed witness confirmations. In the context of patent law, proof of conception and reduction to practice often relies entirely on these laboratory records. Digital systems are essential here; ELNs provide automatic timestamping, secure storage, and integrated witness sign-off features, which are far more robust than traditional paper notebooks for establishing the chain of custody and authenticity of a discovery.
The process of managing IP extends beyond internal documentation to include a continuous review of the competitive and patent landscape. Researchers must regularly perform freedom-to-operate (FTO) searches. This ensures that their current research pathways do not inadvertently infringe upon existing patents held by competitors. This iterative intellectual property awareness guides research decisions, steering the industrial research lab toward white spaces in the technology map where proprietary discoveries can be more easily protected.
Furthermore, strategic decisions must be made regarding the type of protection—patent, trade secret, or copyright—that is most appropriate for a given invention. Not all valuable discoveries are patented. Some core processes or unique data algorithms may be better protected as trade secrets, which requires an additional layer of internal security and access control. This multi-faceted approach to managing IP transforms the industrial research lab from a mere cost center into a powerful engine of economic value creation. The clear establishment of IP protocols within the industrial research lab must be communicated to and understood by all technical staff, ensuring compliance across the organization.
Advancing scientific scale in the industrial environment
The transition of scientific discovery from the benchtop to industrial application requires a dedicated focus on scale, efficiency, and compliance. The modern industrial research lab operates under a set of rigorous standards and governance structures designed to maximize reproducibility and economic return. From the strategic design of flexible infrastructure to the adoption of validated high-throughput screening platforms, every operational decision is calibrated toward accelerating the innovation pipeline while rigorously adhering to regulatory and quality standards. Mastery of the complexities involved in managing IP, implementing robust QC systems, and executing comprehensive risk management protocols are the defining factors that enable an industrial research lab to successfully drive large-scale, cross-sector innovation. These principles are indispensable for laboratory professionals committed to impactful scientific output.
Frequently asked questions (FAQ)
What role do QC systems play in modern industrial research lab operations?
Robust QC systems are non-negotiable in an industrial research lab as they guarantee the reliability and integrity of all scientific and operational data. These systems encompass a variety of protocols, including instrument calibration, method validation, and the strict use of quality reference materials. For high-volume work, such as with high-throughput screening platforms, QC incorporates automated checks like the Z'-factor to assess assay quality on every plate. The primary objective is to ensure that results are reproducible and accurate across different personnel, instruments, and time points, which is a fundamental requirement for regulatory submissions (e.g., FDA or EMA) and successful technology transfer to manufacturing or clinical phases. Consistent adherence to these systems mitigates the risk of costly experimental failures and protects the organization’s scientific reputation.
How does strategic risk management ensure operational continuity for cross-sector R&D labs?
Risk management provides a comprehensive framework to safeguard the high-value operations of cross-sector R&D labs. This involves assessing and mitigating diverse threats, including laboratory safety hazards, regulatory non-compliance, and operational downtime. For a multi-disciplinary industrial research lab, this means developing integrated safety protocols that account for chemical, biological, and physical risks across different specialty areas. A key component of strategic risk mitigation is the creation and regular testing of disaster recovery and business continuity plans, ensuring critical experimental data and specialized equipment are protected. Effective risk mitigation protocols are essential for maintaining the continuous flow of innovation and protecting the significant financial investment in complex industrial research lab infrastructure.
What are the key elements of managing IP when accelerating prototyping in an industrial research lab?
Managing IP is a continuous process that begins at the earliest stages of accelerating prototyping. The core element is meticulous, non-repudiable documentation, typically secured through digital tools like Electronic Laboratory Notebooks (ELNs) that automatically timestamp and require witnessed sign-offs. This documentation is critical for establishing inventorship and establishing a clear date of conception for patent applications. Furthermore, the strategy involves proactively steering the research toward patentable novelty by conducting continuous freedom-to-operate searches to avoid infringing on existing intellectual property. In the context of rapid iteration and prototyping, clear IP guidelines ensure that every novel iteration or design refinement is properly recorded and assessed for its commercial protection potential before being publicly disclosed.
What distinguishes the design requirements for industrial labs versus academic facilities?
The primary distinction in designing industrial labs is the mandate for scalability, regulatory compliance, and economic efficiency. Academic facilities often prioritize flexibility for diverse, exploratory projects, while an industrial research lab is designed with clear process workflows, segregated zones for specific operational stages (R&D vs. QC), and rigorous environmental controls required for GLP or GMP compliance. Industrial designs integrate utility infrastructure with automation and high-throughput screening platforms, ensuring power, data lines, and specialized gases are configured for continuous, high-volume operation. The infrastructure decisions are directly driven by risk management and the long-term goal of high-volume product creation, necessitating durable, easy-to-clean materials and robust data management systems like LIMS for auditability.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.














