Chemical reactions can be studied at different levels: At the level of individual atoms and molecules, new compounds can be designed. At the level of tiny particles on the nano and micrometer scale, one can understand how catalyst materials influence chemical reactions. In order to use chemical reactions in industry, however, it is necessary to look at the macroscopic scale.
Typically, different approaches are used for each area. But this is not sufficient for complex chemical reactions on catalyst surfaces. At University of Technology (TU) Wien (Vienna), an important step has now been taken: for the first time, it is possible to connect all levels from the microscopic to the macroscopic in order to describe a technologically important chemical reaction under realistic conditions. The results have now been published in the scientific journal Nature Communications.
Isomers: same composition, different molecules
Many molecules come in different variants: The same set of atoms can be arranged in different ways, which are then referred to as "isomers." It is important to distinguish between these isomers—for example, a certain isomer of the hydrocarbon butene is favorable for fuel production, but another butene variant is preferred for polymer manufacturing. Producing exactly the desired isomers or converting one isomer into another is a tricky task that can be achieved with very specific catalysts.
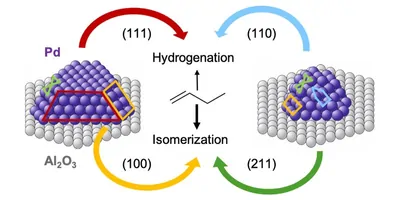
"A particularly important catalyst for such processes is palladium," says professor Günther Rupprechter from the Institute of Materials Chemistry at TU Wien. "Normally, palladium is placed on a surface in the form of tiny nanocrystals. Certain molecules then bind to these granules, and this enables the chemical reaction."
It is a well-known fact that the particle size is often crucial for a specific catalytic function, but mostly there has been no detailed rationalization of how this works. "It is impossible to create a full-scale quantum-chemical model of these particles on a computer, because they simply consist of too many atoms," says Dr. Alexander Genest, the first author of the current study. "We therefore have to find alternatives to combine the different methods to study chemical catalysis."
Realistic conditions instead of idealized systems
The research team at TU Wien and its cooperation partners from Singapore, Alicante, and Munich chose a complex but important reaction for their investigations: The isomerization of alkenes. "This is particularly challenging because there are several reaction pathways that play a role at the same time," says Rupprechter. "It was important for us to study the reaction under realistic conditions: In previous basic research, reactions were often analyzed in (ultra-)high vacuum, at low temperatures. But in an industrial setting, you have to deal with completely different parameters. We therefore wanted to find out how this isomerization takes place at atmospheric pressure and 100°C."
The team started at the level of atoms and molecules: "With the help of density functional theory, we can model elementary reaction steps of the molecules that attach to various facets of the palladium crystals," says Genest. These calculations yield parameters for so-called microkinetic models, which can be used to predict the dynamics of chemical reactions on a much larger time scale on a computer. And from these results, in turn, it is then possible to infer the total amount of desired chemical products that will be present after a certain time at certain parameters.
"The model calculations agree very well with our experimental measurements, not only qualitatively but also quantitatively," emphasizes Rupprechter. "This is an important breakthrough—such agreement was not possible like this before." Now it can be explained in detail why various sizes of palladium particles have different effects on the chemical processes: Large particles have smooth surfaces, while smaller ones are more round and stepped. The arrangement of the palladium atoms in alternative geometries influences the reaction energy and thus the catalytic behavior.
Optimal results instead of just trial and error
"When you optimize a chemical process in industry, you often have to rely on trial and error," says Rupprechter. "Which external parameters should be chosen? Which catalysts do you use—and in what form? These are questions that could hardly be answered on a theoretical level until now." Usually, multiple variants are tested and then the most successful one is chosen. But if a process is then supposed to be scaled up from laboratory scale to industrial scale, completely different parameters may be required.
"We have now shown that you can comprehensively understand such processes if you link several time- and length scales," says Genest. "This approach is of course also applicable to many other catalytic reactions." In the chemical industry, it should thus become possible to optimize chemical manufacturing processes through computer modeling and at the same time reduce expensive and time-consuming benchmarking to a minimum.
- This press release was originally published on the Vienna University of Technology website