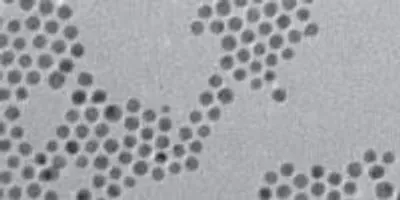
This TEM shows gold–copper bimetallic nanoparticles used as catalysts for the reduction of carbon dioxide, a key reaction for artificial photosynthesis.Berkeley LabThe excessive atmospheric carbon dioxide that is driving global climate change could be harnessed into a renewable energy technology that would be a win for both the environment and the economy. That is the lure of artificial photosynthesis in which the electrochemical reduction of carbon dioxide is used to produce clean, green and sustainable fuels. However, finding a catalyst for reducing carbon dioxide that is highly selective and efficient has proven to be a huge scientific challenge. Meeting this challenge in the future should be easier thanks to new research results from Berkeley Lab.
This TEM shows gold–copper bimetallic nanoparticles used as catalysts for the reduction of carbon dioxide, a key reaction for artificial photosynthesis.Berkeley LabThe excessive atmospheric carbon dioxide that is driving global climate change could be harnessed into a renewable energy technology that would be a win for both the environment and the economy. That is the lure of artificial photosynthesis in which the electrochemical reduction of carbon dioxide is used to produce clean, green and sustainable fuels. However, finding a catalyst for reducing carbon dioxide that is highly selective and efficient has proven to be a huge scientific challenge. Meeting this challenge in the future should be easier thanks to new research results from Berkeley Lab.
Peidong Yang, a chemist with Berkeley Lab’s Materials Sciences Division, led a study in which bimetallic nanoparticles of gold and copper were used as the catalyst for the carbon dioxide reduction. The results experimentally revealed for the first time the critical influence of the electronic and geometric effects in the reduction reaction.
“Acting synergistically, the electronic and geometric effects dictate the binding strength for reaction intermediates and consequently the catalytic selectivity and efficiency in the electrochemical reduction of carbon dioxide,” Yang says. “In the future, the design of carbon dioxide reduction catalysts with good activity and selectivity will require the careful balancing of these two effects as revealed in our study.”
Yang, who also holds appointments with the University of California (UC) Berkeley and the Kavli Energy NanoSciences Institute at Berkeley, is a leading authority on nanoparticle phenomena. His most recent research has focused on nanocatalysts fashioned from metal alloys rather than a single metal such as gold, tin or copper.
“By alloying, we believe we can tune the binding strength of intermediates on a catalyst surface to enhance the reaction kinetics for the carbon dioxide reduction,” he says. “Nanoparticles provide an ideal platform for studying this effect because, through appropriate synthetic processes, we can access a wide range of compositions, sizes and shapes, allowing for a deeper understanding of catalyst performance through precise control of active sites.”
 Nanoscience expert Peidong Yang holds appointments with Berkeley Lab, UC Berkeley and the Kavli Energy NanoSciences Institute at Berkeley.Roy KaltschmidtIn addition, Yang says, nanoparticle as catalysts have high surface-to-volume and surface-to-mass ratios that are advantageous for achieving high catalytic activity. For this new study, uniform gold–copper bimetallic nanoparticles with different compositions were assembled into ordered monolayers then observed during carbon dioxide reduction.
Nanoscience expert Peidong Yang holds appointments with Berkeley Lab, UC Berkeley and the Kavli Energy NanoSciences Institute at Berkeley.Roy KaltschmidtIn addition, Yang says, nanoparticle as catalysts have high surface-to-volume and surface-to-mass ratios that are advantageous for achieving high catalytic activity. For this new study, uniform gold–copper bimetallic nanoparticles with different compositions were assembled into ordered monolayers then observed during carbon dioxide reduction.
“The ordered monolayers served as a well-defined platform that enabled us to better understand their fundamental catalytic activity in carbon dioxide reduction,” Yang says. “Based on our observations, the activity of the gold-copper bimetallic nanoparticles can be explained in terms of the electronic effect, in which the binding of intermediates can be tuned using different surface compositions, and the geometric effect, in which the local atomic arrangement at the active site allows the catalyst to deviate from the scaling relation.”
The effects Yang and his colleagues observed for gold-copper bimetallic nanoparticles should hold true for other carbon dioxide reduction catalysts as well.
“We expect the effects we observed to be universal for a wide range of catalysts, as evidenced in other areas of catalysis such as the hydrogen evolution and oxygen reduction reactions,” says Dohyung Kim, a member of Yang’s research group and a collaborator in this study. “The factors we have identified are based on the solid concept of electrocatalysis.”
Knowing the influence of the electronic and geometric effects makes it possible to deduce how intermediate products in the reduction of carbon dioxide, such as carboxylic acid and carbon monoxide, will interact with the surface of a newly proposed catalyst and thereby provide the means for predicting the catalyst’s performance. Coupled with the exceptional structuring of active catalytic sites made possible by the use of nanoparticles, the path is paved, Yang and his colleagues believe, for unprecedented improvements in electrochemical carbon dioxide reduction.
“My group is now using the insights gained from this study in the design of next generation carbon dioxide reduction catalysts,” Yang says.
A paper describing this research has been published in Nature Communications entitled “Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles.” Yang is the corresponding author and Kim is the lead author. The other co-authors are Joaquin Resasco, Yi Yu and Abdullah Mohamed Asiri.
Additional Information
For more about the research of Peidong Yang go here