Forensic laboratories constantly face the challenge of reducing case backlogs. To address this, many are adopting rapid DNA kits and portable forensic instruments while maintaining rigorous standards of accuracy. These technologies have fundamentally altered the landscape of preliminary biological analysis within the justice system. Distinct scientific processes were traditionally tethered to benchtop equipment. Now, they occur at the crime scene or booking station without sacrificing the fidelity required for investigative leads. Laboratory professionals must navigate the technical nuances and validation requirements of these tools. They must also manage the data integration challenges presented by decentralized analysis.
Operating mechanics of rapid DNA kits
Miniaturization of capillary electrophoresis enables the generation of full genetic profiles in under two hours.
Rapid DNA kits function on a "sample-in, profile-out" basis, automating the complex steps of the forensic workflow—lysis, purification, amplification, and detection—within a single, closed microfluidic cartridge. Unlike traditional methods requiring separate rooms and instruments to prevent contamination during Polymerase Chain Reaction (PCR), these systems contain the chemistry within sealed channels. The instrument utilizes lyophilized reagents that rehydrate upon run initiation, reducing the need for cold chain storage which often complicates field logistics.
During the process, pneumatic, fluidic, and thermal subsystems manage the sample through several critical stages:
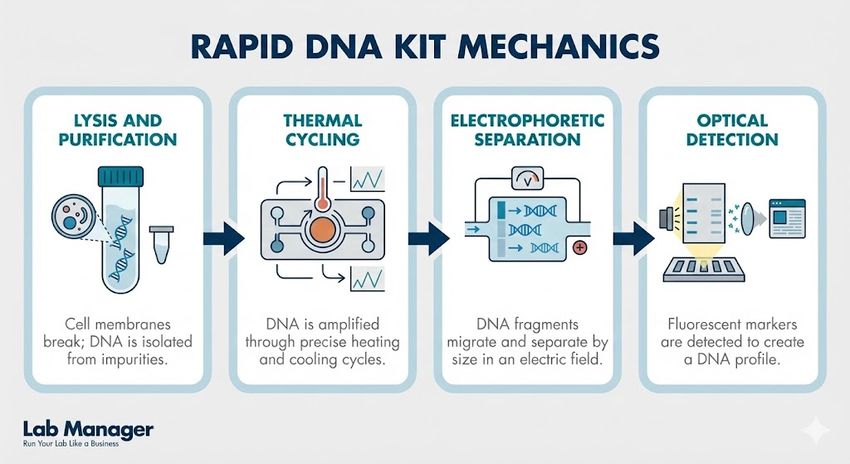
Modern Rapid DNA technology condenses complex benchwork into a single, automated pipeline.
GEMINI (2025)
- Lysis and purification: Chemical disruption of cells followed by the isolation of DNA from potential inhibitors.
- Thermal cycling: Rapid heating and cooling cycles designed to amplify specific STR loci using onboard reagents.
- Electrophoretic separation: Utilizing high voltage to separate DNA fragments by size within a miniaturized capillary array.
- Optical detection: Laser excitation of fluorescent dyes to capture raw data for immediate algorithmic analysis.
A laser detection system then reads the fluorescently tagged fragments, and onboard expert system software analyzes the raw data to produce an electropherogram. This automation significantly reduces the hands-on time required by analysts, though it necessitates a shift in focus toward instrument validation and quality assurance monitoring.
The Federal Bureau of Investigation (FBI) and the Scientific Working Group on DNA Analysis Methods (SWGDAM) have established specific standards for these devices. Compliance ensures that profiles generated by rapid DNA kits meet the criteria for upload to the National DNA Index System (NDIS), provided they are used in approved environments like booking stations or accredited laboratories.
Applications of portable forensic instruments in field testing
Field-deployable devices extend beyond biological analysis to provide immediate chemical identification and substance verification.
While DNA analysis addresses identity, other portable forensic instruments target the identification of illicit narcotics, explosives, and hazardous materials. Handheld Raman and Fourier Transform Infrared (FTIR) spectrometers have become standard assets for rapid substance analysis. These devices allow operators to scan substances through transparent packaging, minimizing exposure to potent opioids like fentanyl. Raman spectroscopy utilizes laser light scattering to identify molecular fingerprints, while FTIR measures the absorption of infrared light to determine chemical composition.
Mass spectrometry has also migrated from the bench to the backpack. High-pressure mass spectrometry (HPMS) devices can now operate near ambient pressure, allowing for the detection of trace levels of chemicals in solid, liquid, or vapor phases. These tools provide laboratory professionals with preliminary data that helps triage samples before they arrive at the central facility.
Comparison of common portable forensic technologies
Technology | Primary Mechanism | Best Application | Laboratory Correlation |
|---|---|---|---|
Rapid DNA | Microfluidic STR Typing | Reference samples (buccal swabs) | Matches traditional STR profiles |
Handheld Raman | Laser Inelastic Scattering | Analysis through glass/plastic containers | Confirmatory benchtop Raman/GC-MS |
Handheld FTIR | Infrared Absorption | Powders and solids (direct contact) | Confirmatory benchtop FTIR |
Mobile Mass Spec | Ion Mass-to-Charge Ratio | Trace vapor and complex mixtures | LC-MS/GC-MS |
Integrating these portable forensic instruments requires rigorous performance checks. The National Institute of Standards and Technology (NIST) provides reference libraries and validation protocols that laboratories must adapt for mobile equipment to ensure field results align with accredited laboratory findings.
Integrating data from portable forensic instruments and rapid DNA
Adopting remote testing devices requires adjustments to traditional laboratory information management systems (LIMS) and personnel roles.
The successful deployment of rapid DNA kits and portable forensic instruments depends heavily on connectivity and data governance. Laboratory professionals act as the gatekeepers for data entering the ecosystem from the field. Innovations in LIMS now allow for "reachback" capabilities, where a field operator transmits raw data from a portable device to the central laboratory for immediate interpretation by a qualified analyst. This hybrid approach maximizes the speed of the instrument while retaining the interpretive expertise of the forensic scientist.
Laboratories must establish distinct workflows for samples processed in the field versus those processed in-house. A critical innovation involves the direct integration of mobile device outputs into the laboratory's secure network. When a rapid DNA kit generates a CODIS-eligible profile, the data packet requires secure transmission protocols to prevent tampering or corruption. Similarly, chemical spectra from handheld units must sync with central libraries to ensure the device software remains current with emerging drug analogs. This digital infrastructure shifts the laboratory professional’s role from purely distinct sample processing to comprehensive system oversight and data validation.
Validation standards for rapid DNA kits and field devices
Admissibility relies on adherence to strict validation guidelines established by oversight bodies and accrediting agencies.
Validation remains the cornerstone of forensic science, and rapid DNA kits face intense scrutiny regarding their reliability compared to standard capillary electrophoresis. Validation studies must demonstrate that the portable instruments perform robustly under varying environmental conditions, such as fluctuating temperatures or humidity levels found at crime scenes. The Organization of Scientific Area Committees (OSAC) for Forensic Science drafts standards that guide these developmental and internal validation studies.
Key validation parameters for ensuring the legal defensibility of portable instrument data include:
- Environmental robustness: Verifying consistent performance across wide ranges of temperature, humidity, and vibration typical of field settings.
- Concordance: Confirming that results generated in the field match those produced by gold-standard benchtop instruments.
- Sensitivity limits: Establishing reliable lower limits of detection to prevent false negatives in low-yield samples.
- Contamination control: Testing the integrity of closed cartridges to ensure no sample-to-sample carryover occurs during rapid processing.
For portable forensic instruments used in chemical analysis, false positives and negatives present significant legal risks. Laboratory directors must implement quality control programs that include regular calibration checks using certified reference materials. Furthermore, the limitations of the technology must be clearly defined in standard operating procedures. For instance, knowing that Raman spectroscopy struggles with fluorescent samples or dark materials prevents field errors.
Validation also extends to the specific swab or substrate types used with rapid DNA kits. While initially approved primarily for reference buccal swabs, ongoing research aims to validate these systems for crime scene samples, such as blood or touch DNA. However, technical challenges regarding stochastic effects and low template DNA in unsupervised field settings currently limit this application for NDIS upload.
Future developments in rapid DNA kits and portable forensic instruments
New developments in sensor technology and bioinformatics promise to further shrink the footprint of forensic analysis tools.
As rapid DNA kits evolve, the industry anticipates a move toward higher multiplexing capabilities, potentially including phenotypic markers or kinship analysis within the rapid timeframe. Simultaneously, portable forensic instruments for chemical analysis are incorporating artificial intelligence to better deconvolve complex mixtures in real-time, reducing the "no match" results that often delay investigations. Laboratory professionals will remain essential in defining the scope of use for these tools, ensuring that the pursuit of speed never compromises the scientific rigor required by the justice system.
Strategic value of rapid DNA kits and portable forensic instruments
The integration of rapid DNA kits and portable forensic instruments into the forensic ecosystem represents a permanent shift toward decentralized analysis. These tools relieve pressure on central laboratories by filtering out non-probative samples and providing immediate investigative intelligence. However, the reliability of these devices rests entirely on the robust validation, quality assurance, and data management protocols established by laboratory professionals. By embracing these technologies as extensions of the laboratory rather than replacements, forensic scientists ensure that justice systems benefit from both speed and unassailable scientific accuracy.
FAQ
What distinguishes rapid DNA kits from traditional DNA analysis methods?
Rapid DNA kits automate the entire DNA profiling process—including extraction, amplification, and detection—within a single instrument in under two hours, whereas traditional methods involve separate manual steps and instruments taking significantly longer.
Are results from portable forensic instruments admissible in court?
Results typically serve as presumptive tests for investigative leads, though some jurisdictions accept them as evidence if the instrument undergoes rigorous validation and the operator maintains strict chain of custody protocols.
Can rapid DNA kits analyze mixed samples effectively?
Most current rapid DNA kits are optimized for single-source reference samples, as the automated expert systems may struggle to deconvolve complex mixtures without human analyst intervention.
How do laboratories ensure the accuracy of field-based instruments?
Laboratories implement strict quality assurance programs that include regular calibration with known standards, proficiency testing for operators, and periodic internal validation of the devices against benchtop gold standards.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.














