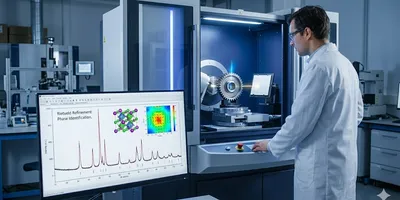
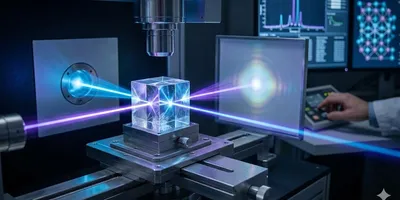
x-ray crystallography

Work could lead to the development of new ways of making important compounds like drugs and electronic materials

With the aid of X-ray crystallography, researchers at the University of Michigan have revealed the structures of two closely related enzymes that play essential roles in the body's ability to metabolize excess lipids, including cholesterol.

Facing a challenge akin to solving a 1,000-piece jigsaw puzzle while blindfolded—and without touching the pieces—many structural biochemists thought it would be impossible to determine the atomic structure of a massive cellular machine called the nuclear pore complex (NPC), which is vital for cell survival.