Part 1: What is a centrifuge used for?
Centrifuges are used in various laboratories to separate fluids, gases, or liquids based on density. In research and clinical laboratories, centrifuges are often used for cell collection, organelle purification, virus purification, protein purification, and nucleic acid purification.
Centrifuges have a long history, with the first commercial centrifuge unveiled in 1864. Antonin Prandtl developed a dairy centrifuge for separating cream from milk. Five years later, in 1869, Friedrich Miescher was the first person to use a centrifuge in a lab to isolate a cell organelle. Learn more about the history of centrifuges with this infographic.
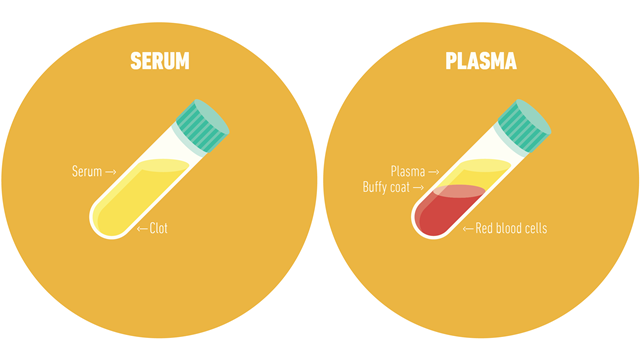
An example of centrifuge use in a clinical setting is for the separation of whole blood components. Different assays necessitate serum or plasma, which may be obtained with centrifugation.
Serum is obtained by letting a whole blood sample clot at room temperature. The sample is then centrifuged, and the clot is removed, leaving a serum supernatant.
Unlike serum, plasma is obtained from whole blood that is not left to clot and contains serum along with clotting factors. To obtain plasma, a whole blood sample is collected in tubes treated with anticoagulants. Following centrifugation, cells are removed, leaving plasma supernatant.
Part 2: How do centrifuges work?
Principles of centrifugation
What does a centrifuge do?
A centrifuge is used to separate particles suspended in a liquid according to particle size and density, viscosity of the medium, and rotor speed.
Within a solution, gravitational force will cause particles of higher density than the solvent to sink, and those less dense than the solvent to float to the top. Centrifugation takes advantage of even minute differences in density to separate particles within a solution.
As the rotor spins around a central axis, it generates a centrifugal force acting to move particles away from the axis of rotation. If the centrifugal force exceeds the buoyant forces of liquid media and the frictional force created by the particle, the particles will sediment.

Part 3: How to choose a centrifuge
Centrifuge size
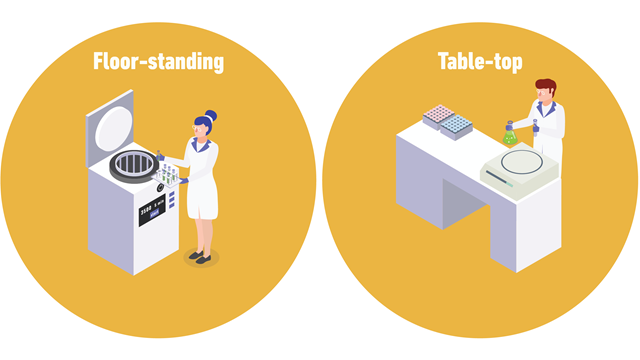
Centrifuges are available as various benchtop or floor-standing models.
Floor-standing models offer greater sample capacity and can achieve high speeds. Superspeed centrifuges can achieve a maximum g-force (relative centrifugal force, RCF) of over 70,000 x g, and ultracentrifuges often used for DNA or RNA fractionation, can achieve up to 1,000,000 x g. For large-capacity, low-speed applications, low-speed centrifuges reaching approximately 7000 x g are available.
Benchtop models have a smaller footprint, and general-purpose models are ideal for a wide range of applications. There are many benchtop models available, including high-speed, microcentrifuge, clinical, and cell washer models. Clinical benchtop models and cell washers typically operate at lower speeds and are suited to diagnostic applications, and washing debris from red blood cells.
Types of centrifuges
Understanding the different types of centrifuges goes beyond the basic classification of benchtop and floor models. Here are some other types of centrifuges that can be classified as either benchtop or floor but have other characteristics that differentiate them.
Refrigerated centrifuges
Designed to maintain a constant temperature for ensuring the viability of heat-sensitive samples, refrigerated centrifuges are a staple in many labs. They are frequently used in biology, biochemistry, and clinical laboratories.
Vacuum centrifuges
Also called vacuum concentrators or centrifugal evaporators, vacuum centrifuges are a unique type of centrifuge that pull a vacuum within the chamber during centrifugation. Rather than separating components based on density as a standard centrifuge does, centrifugal evaporators concentrate samples by evaporating solvents. Much like a freeze dryer, pulling a vacuum in the chamber lowers the boiling point of the sample solvents, allowing the solvents to evaporate faster and leave concentrated samples. Vacuum centrifuges are common in pharmaceutical drug development, genomic, and proteomic research, where they are used in purifying DNA/RNA and proteins.
Microcentrifuges
Designed to process small sample volumes, microcentrifuges offer high-speed centrifugation on a small scale. Their compact footprint makes them suited for labs where bench space is limited. They are common in molecular biology laboratories to prep DNA/RNA and protein samples.
Ultracentrifuges
As the name implies, ultracentrifuges are designed for ultra-fast spinning. With models able to spin between 60,000 RRPM all the way up to 150,000 RPM, they are suited well for separating very fine particles such as viruses and ribosomes. Ultracentrifuges are often used in medical settings, such as in hematology labs for blood separation and analysis.
Analytical ultracentrifuges
Typically, centrifuges are part of a lab’s sample preparation process to ready samples for analysis. However, analytical ultracentrifuges play an active role in analysis. They are equipped with optical systems such as UV-Vis or fluorescence detection that analyze samples in real-time during centrifugation. They can provide detailed information on the characteristics of the molecules, such as sedimentation velocity.
Centrifuge rotor types
There are two very common rotor designs: fixed angle, and swinging bucket. The fixed-angle rotor is designed to hold tubes in a fixed position at a fixed angle relative to the vertical axis of rotation (up to about 45°). Centrifugation will cause particles to sediment along the side and bottom of the tube. The swinging bucket design allows the tubes to swing out from a vertical resting position to become parallel to the horizontal during centrifugation. As a result, sediment will form along the bottom of the tube.
Fixed angle rotors are ideal for pelleting applications either to remove particles from a suspension and discard the debris or to recover the pellet, whereas swinging bucket rotors are best for separating large volume samples at low speeds and resolving samples in rate-zonal (density) gradients.

Centrifuge speed

Centrifuges may be classified based on maximum speeds, measured as revolutions per minute (RPM). Speeds range from 0-7,500 RPM for low-speed centrifuges, all the way to 20,000 RPM or higher.
Centrifuge rotor speed is often expressed as RCF in units of gravity (x g) for various procedures. However, many centrifuges display speed as revolutions per minute (RPM), necessitating conversion to ensure the correct experimental conditions. The following formula is used to convert RPM to RCF, where R is the rotor radius (cm) and S is the speed (RPM):
g = (1.118 x 10-5) R S2
Centrifuges for different applications
It is essential to select a centrifuge that is suited to the specific application. When purchasing a centrifuge, it is important to consider the following questions:
- What sample volumes are you working with? For processes involving large or varying volumes, a floor-standing model with higher capacity and different rotor configurations may be the best solution.
- Are samples temperature-sensitive? If so, a centrifuge with refrigeration and temperature control options is required.
- Will the centrifuge be used to process clinical or blood banking samples? Cell washers or clinical models are available for these specific applications.
- How much laboratory space is available vs the centrifuge footprint?
- What is the maximum g-force the centrifuge is capable of generating? Low-speed centrifuges are ideal for separating whole cells, while ultracentrifuges are necessary for separating DNA and RNA.
Part 4: How to use a centrifuge safely
Ensure a sturdy, level work surface
Always ensure the centrifuge is on an appropriate surface prior to operation.
Balance the centrifuge
Running an unbalanced centrifuge may cause significant damage, and injure the operator and other laboratory personnel. The total mass of each tube should be as close as possible- this becomes increasingly important at very high rotor speeds. Balancing masses to the nearest 0.1 gram is advisable, and it is important to balance tubes by mass, not volume. For example, do not balance a sample consisting of liquid with a higher or lower density than water with an equal volume of water.
Do not open the lid while the rotor is moving
Many centrifuges have a “safety shutoff.” However, this will only stop power to the rotor, which will still spin due to its own inertia for some time until it is slowed to a stop by friction.
If the centrifuge is wobbling or shaking, pull the plug
A little vibration is normal, but excessive amounts can mean danger. First, double-check that the tubes are correctly balanced. If this does not resolve the issue, do not operate the centrifuge until it has been serviced by the manufacturer or dealer.
Part 5: How do you balance a centrifuge?
Why do you need to balance a centrifuge?
Prior to starting the centrifuge, it is necessary to load it correctly. Balancing the centrifuge prevents potential damage to the instrument, and is crucial for safe operation.
How to balance a centrifuge
- Ensure all sample tubes are evenly filled. If additional tubes are required for balancing, fill them with water or a liquid of similar density to the sample, and ensure the mass is balanced to the nearest 0.1 grams.
- For each tube inserted in the rotor, add a tube of equal weight directly opposite it. This will ensure the center of gravity remains in the center of the rotor.
- Rotate the rotor 90° and add two additional tubes directly opposite one another.
- Repeat.

How to balance 3 tubes, 5 tubes, or 7 tubes in a centrifuge with 12 positions
There are two ways to balance three tubes. The first option is to insert three sample tubes next to each other, and create three balance tubes to be situated directly across from the sample tubes.
Alternatively, three sample tubes may be spaced evenly around the rotor.

To balance five tubes, create one balance tube and place two sets of three tubes across from each other.

To balance seven tubes, create one balance tube and place two sets of four tubes across from each other.

Part 6: How do you maintain a centrifuge?
Q: What are the basic steps to maintain a centrifuge properly?
A: To keep your centrifuge in top condition, follow these simple steps:
- Regularly lubricate the centrifuge, especially the O-rings which protect against sample leakage.
- Ensure that any threaded components are cleaned and lubricated with approved grease to prevent cross-threading and corrosion.
- Train all users on proper centrifuge operation, including bucket placement, tube balancing, and adhering to rotor speed guidelines.
Q: How do you inspect a centrifuge for wear and tear?
A: Regular inspections should focus on:
- Checking for scratches or chemical exposure effects on the rotor.
- Noting any unusual noise, vibration, shaking, or grinding, and stopping the unit immediately if these occur.
Q: What is the recommended procedure for cleaning a centrifuge?
A: For cleaning, use these guidelines:
- Clean regularly with neutral solutions like alcohol or alcohol-based disinfectants.
- Use a soft cloth for rotors and accessories.
- Daily cleaning should include the interior, rotor chamber, and any electronic components like touchscreens and keypads.
- Be mindful of the types of samples used and any specific cleaning protocols required for spills.
Q: Are there any special considerations for training new users in centrifuge maintenance?
A: Yes, it’s important to implement broad and clear cleaning procedures. Emphasize to new staff the importance of decontaminating the area, regardless of the risk group of the work being done. When training new users, it may be useful to implement clear, broad cleaning procedures for the centrifuge. Hiba Shamma, a lab coordinator at Battelle Organization, says that “When I give them [new lab staff] orientation, [I tell them] ‘Hey, even if you’re not doing any Risk Group 1, Risk Group 2 work—even if it’s protein work you’re doing—you have to decontaminate your area with bleach and isopropanol.”
Understanding the diverse types of centrifuges, from standard benchtop models to sophisticated ultracentrifuges and analytical ultracentrifuges, is essential for any laboratory operation. Whether you're separating delicate biomolecules or analyzing complex macromolecular structures, the right centrifuge can significantly enhance the efficiency and accuracy of your work. Remember, proper operation and maintenance are key to the longevity and performance of your centrifuge.












