Battery usage is expanding across various technologies, from consumer electronics to electric vehicles and grid-level energy storage. Battery material analysis is becoming increasingly critical as the industry is expected to grow by 25 percent annually through 2030, reaching over $400 billion globally.
Manufacturers face increasing competition to reduce battery costs and size while improving efficiency, longevity, and charging time. Safety and stability concerns are significant due to the potential chemical reactivity of batteries, highlighted by incidents of explosions and fires. Sustainability and supply chain issues, such as managing battery waste and the limited availability of lithium, also pose challenges.
To meet these demands, understanding battery performance under real conditions and minimizing waste during R&D and manufacturing are crucial, requiring precise and predictive battery material analysis tools.
Optimizing battery electrode performance with rheology
Battery electrodes—anodes and cathodes—are produced by mixing active material, conductive additive, binder, and solvent into a slurry, and coating this slurry onto a metal current collector. Slurry formulations must be optimized to achieve the desired behavior during the coating process, ensuring a uniform electrode. Traditionally, viscometers have been used to assess these slurries. However, measuring slurry viscosity at a single shear rate often fails to detect variations, leading to inconsistent and uneven coatings, which compromise battery performance.
Rheometers, on the other hand, provide a complete view of flow behavior over the full range of shear rates relevant to electrode production. In figure 1, two anode slurries appear similar in the viscometer’s measurement range, but their viscosities are much lower at the high shear rates used during coating. These data show that Slurry 2 is thicker at the low shear rates used by manufacturers and can be more evenly distributed, suggesting better electrode performance.
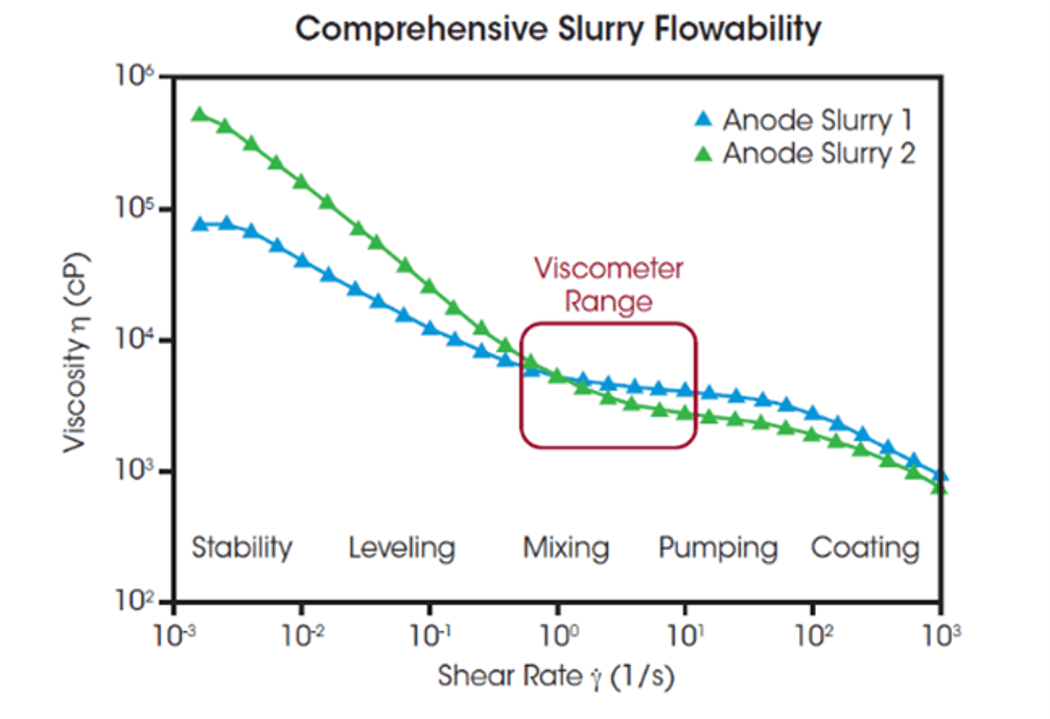
Figure 1
Battery manufacturers are using rheological measurements to inform decisions outside of the R&D lab as well. In the QC lab, simple, easy-to-use rheometers can provide the critical measurements to catch electrode failures before they happen, thereby reducing scrap and ensuring successful coating for every batch of slurry.
Rheology is a powerful tool to optimize the coating process, but successful electrodes must also achieve an even distribution of conductive material. This even coating facilitates ion/electron transfer with the active material to maximize capacity and charge/discharge speeds. For many battery scientists, the slurry is a “black box;” they lack visibility to the conductive network in the slurry and can only evaluate the impacts of formulation changes in the finished cell.
Rheology-impedance spectroscopy (rheo-impedance) can provide new visibility into the slurry “black box” with quantitative measurements of the conductive network while still in the slurry state. These quick measurements can reveal the impacts of substituting an ingredient in the formulation or changing the mixing protocol. Coupling impedance measurements with rheometric data provides visibility to possible changes during and after the high shear coating process, verifying that the conductive network is maintained in the finished electrode. This information can be used to predict and properly formulate slurries for successful electrodes and optimal battery performance.
In figures 2 and 3, Slurry 1 (left) and Slurry 2 (right) show differing electrical properties under shear. The impedance curves for Slurry 1 are consistent across shear rates indicating a stable conductive network. However, Slurry 2 shows changes in impedance curves, suggesting that increasing shear rate causes higher overall resistance, decreasing the efficacy of the conductive network.
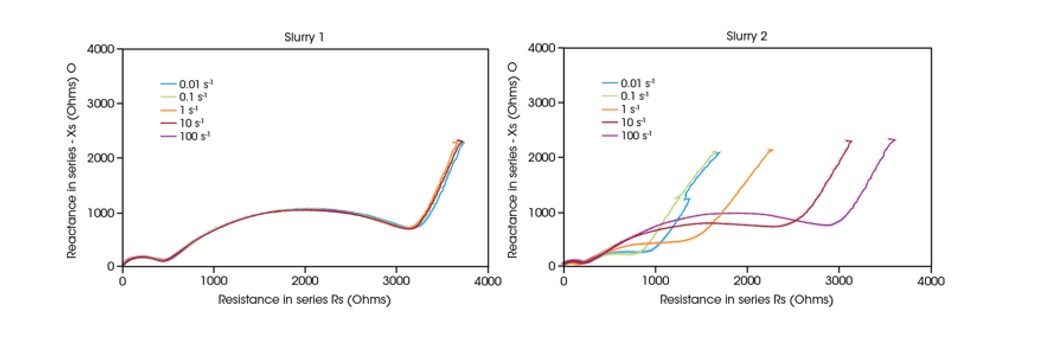
Figures 2 and 3
By combining rheology and impedance spectroscopy, the conductive network can be measured under simulated processing conditions, such as before, during, and after coating, all without needing to physically coat and dry the slurry. This hyphenated technique provides early insight into the critical changes in conductive structure due to shear/flow dynamics and time.
Turning up the heat on battery safety
Thermal analysis techniques, including thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC), are commonly used to determine thermal stability, including the onset of an exothermic reaction and the heat of reactions for battery materials. The lithium salts (such as LiPF6) and organic-based carbonate solvents used in commercially available lithium-ion batteries are known to be highly flammable at high temperatures, emitting toxic gases (such as hydrofluoric acid) during thermal degradation.
Battery developers use TGA to quantify thermal stability, as well as oxidation and thermal degradation. TGA is used to show the temperatures at which battery materials start to degrade. Combining TGA with evolved gas analysis such as infrared spectroscopy (FTIR) or mass spectrometry enables researchers to understand the gases that are produced during thermal degradation and minimize or eliminate toxic gas release during battery thermal runaway.
DSC measures the heat absorbed or released by a sample during heating or cooling over a range of temperatures. DSC provides insights into the heat of reactions, heat capacity, and phase transitions, such as melting point, heat of fusion, and glass transition, enabling researchers to evaluate thermal stability of materials used in their batteries. Understanding the onset temperature and amount of heat released during an exothermic reaction can help battery engineers design thermal management systems and perform safety evaluations of cathode and anode materials in different states of charge.
Whole battery testing to assess battery performance
Evaluating the battery during charge and discharge is crucial for assessing efficiency, quality, and understanding lithium-ion battery chemistry. Batteries are highly dynamic systems with a mixture of electrochemical reactions, chemical reactions, and structural changes occurring during each cycle, producing heat as a byproduct. Electrochemical analysis can only provide information on processes that affect the electrochemical reactions, leaving all other processes (chemical, phase, structural) uncharacterized. In-operando electrochemical microcalorimetry is the leading technique for studying parasitic reactions, which is a blanket term for any side-reactions that can significantly impact the performance and longevity of lithium-ion batteries.
Measuring time-correlated voltage and heat flow signals of a battery under charge and discharge cycles (as shown in figure 4) can reveal the thermal contribution of parasitic reactions.4 Cycling a cell to failure can take many months but emerging in-operando tests with additional thermal insight are able to predict long-term behavior in a matter of weeks. The results help measure cell quality, aid in material formulation, assess additive impacts, study the solid electrolyte interphase (SEI), and predict cycle and calendar life. These data can help researchers accelerate new battery materials development, reduce parasitic activity, and conduct fast screening of cells with high parasitic activity for quality control.
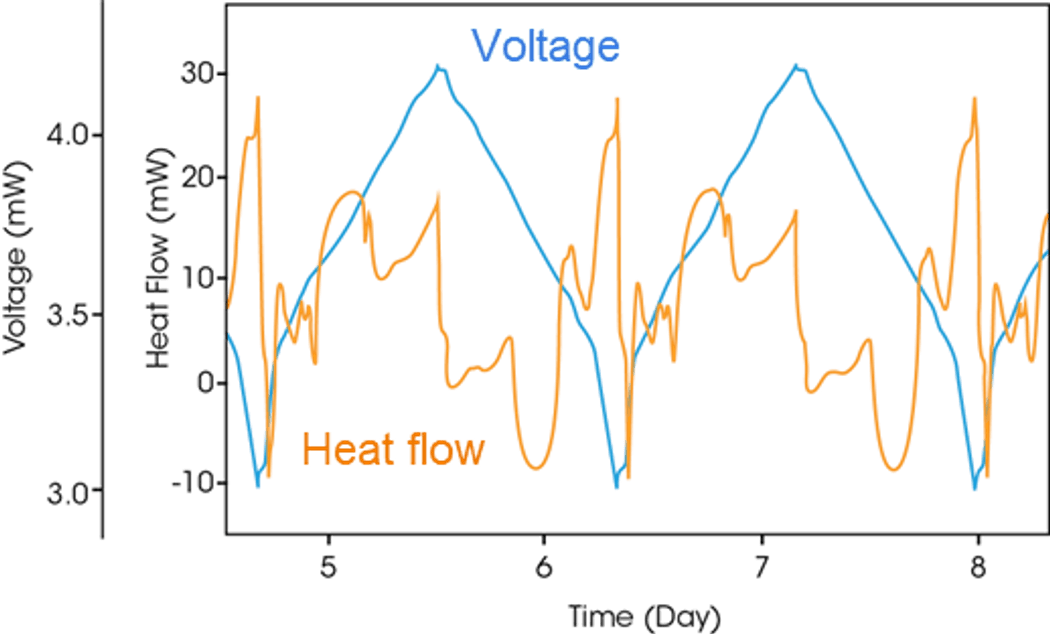
Figure 4
Conclusion
In the race to develop safer, better-performing batteries more efficiently, advanced battery material analysis and analytical testing provide crucial information for improving battery chemistry and manufacturing. Rheology, thermal, and electrochemical calorimetry analysis offer insights ranging from individual material analysis to formulations, materials, and whole-cell battery performance. Embracing the latest technology is helping battery developers bring more durable, safer, and cheaper batteries to market faster.















