The modern scientific environment demands that biological labs evolve beyond mere experimental spaces. They must transform into highly efficient, data-driven, and intrinsically safe ecosystems. This shift requires laboratory professionals to master complex workflows, integrate disparate disciplines, and strategically navigate resource limitations while adhering to global standards for containment and practice. Achieving genuine biosafety leadership within these critical facilities is not just a regulatory requirement. It is a foundational pillar that sustains research integrity and protects personnel, directly influencing the reliability and impact of scientific discoveries.
Strategic lab design and the multidisciplinary mandate
The structure of contemporary research necessitates that biological labs move away from siloed operations toward truly multidisciplinary labs that foster collaboration between diverse scientific fields, such as genomics, proteomics, and computational biology. Effective lab design is the critical initial step in this transition, dictating flow, efficiency, and safety. A strategically designed laboratory optimizes the spatial relationship between wet-bench work areas, instrumentation suites (like those dedicated to advanced imaging), and dry analytical spaces.
Key design principles for modern biological labs:
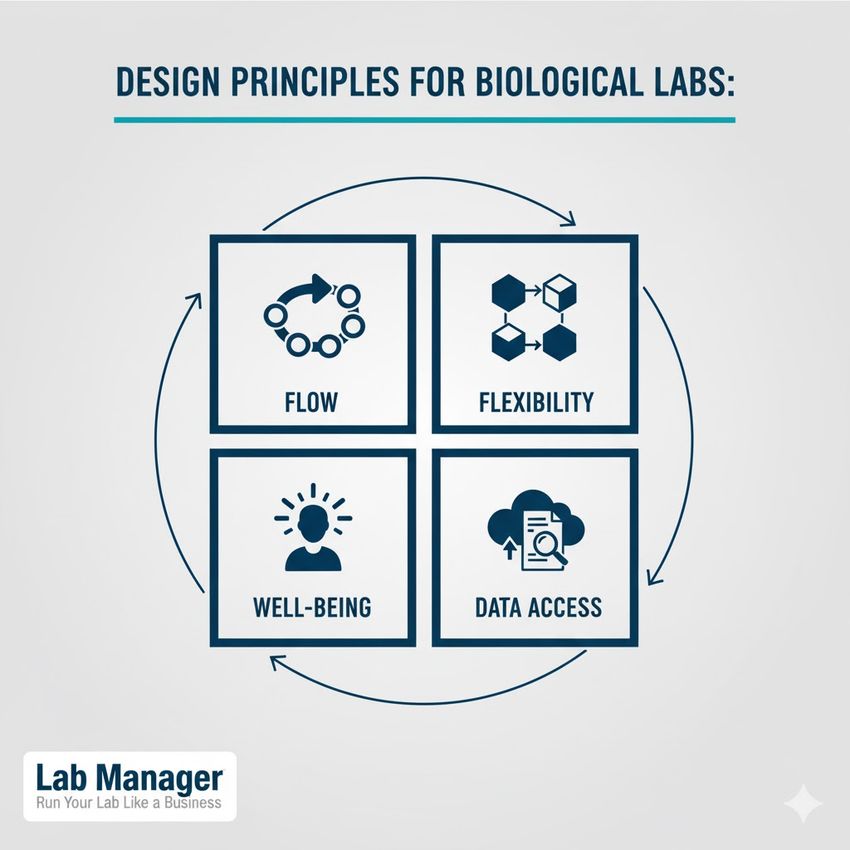
The blueprint for a modern biological lab: These four principles drive efficiency and innovation.
GEMINI (2025)
- Zoning and flow: Establishing distinct zones—dirty, clean, and analytic—minimizes contamination risks and enhances operational efficiency. Dedicated receiving areas for samples, instrumentation cores, and personnel decontamination stations are essential.
- Flexibility and adaptability: Labs should utilize modular furniture and utility connections (power, gas, vacuum, data) to allow for rapid reconfiguration. This flexibility is crucial for accommodating new technologies or shifting research priorities without costly renovations.
- Ergonomics and well-being: Thoughtful design reduces physical strain and human error. Features include height-adjustable benches, non-slip flooring, and ample natural lighting, which contribute positively to alertness and focus, indirectly supporting safety.
- Integration of data infrastructure: High-throughput science generates massive datasets. Lab design must account for robust data connectivity, secure server locations, and dedicated data processing workstations, ensuring seamless integration of experimental results with computational analysis.
In a multidisciplinary lab setting, shared resources must be managed with stringent access controls and scheduling. For example, a centralized flow cytometry core serving immunology, cancer biology, and virology must have standardized operating procedures (SOPs) that mitigate cross-contamination and ensure high-quality data output for all user groups. The physical layout, therefore, directly underpins the operational excellence and long-term viability of the research enterprise.
Financial acumen and budget management for sustainability
Effective operation of biological labs hinges on sound financial planning and resource allocation. Laboratory managers must demonstrate acute budget management skills. They should treat the lab as a complex business unit with fixed overheads, variable project-specific costs, and long-term capital needs. Poor financial oversight can jeopardize research continuity and delay critical projects. Ultimately, it compromises the facility's ability to maintain high safety standards.
The challenge of budget management in scientific settings is magnified by the high cost of specialized reagents, consumables, and maintenance contracts for sophisticated equipment (e.g., mass spectrometers or advanced imaging systems). A proactive budget strategy requires a granular understanding of expenditures and careful forecasting.
Components of strategic laboratory budget management:
Budget Category | Description | Optimization Strategy |
|---|---|---|
Capital expenditure (CapEx) | Acquisition of high-cost, long-life assets (e.g., freezers, sequencing machines). | Centralized, shared instrumentation cores to maximize utilization and reduce duplication across multidisciplinary labs. |
Operational expenditure (OpEx) | Consumables, reagents, utilities, equipment maintenance, and small supplies. | Implement centralized purchasing for volume discounts; adopt "just-in-time" inventory control for expensive reagents to minimize waste. |
Personnel costs | Salaries, benefits, and training for researchers, technicians, and safety personnel. | Invest in highly specialized training (e.g., certification for specific biosafety levels) to increase staff efficiency and minimize errors. |
Compliance and safety | Personal protective equipment (PPE), waste disposal, certification fees, and dedicated safety personnel. | Budget for routine biosafety audits and continuous professional development in biosafety leadership for management staff. |
Successful budget management involves transitioning from reactive spending to a predictive model using sophisticated tracking software. Furthermore, exploring external funding opportunities, such as government grants for infrastructure or industry partnerships for specific assays, helps diversify revenue streams and ensures the long-term sustainability of high-impact research. Financial stability is a prerequisite for maintaining the necessary controls required for compliance with stringent regulations, allowing the lab to focus resources on scientific output while assuring a continuous safety commitment.
The imperative of reproducibility and sample management excellence
Scientific integrity demands that findings be reliable and repeatable. Reproducibility is the cornerstone of trust in science, and its success is intricately linked to meticulous sample management and documentation within biological labs. Failures in this area lead to wasted resources, retracted papers, and a loss of public confidence.
Effective sample management starts at the point of collection and continues through processing, storage, and disposal. A robust Laboratory Information Management System (LIMS) is essential for tracking the provenance, location, and key characteristics of every biological specimen.
Best practices for sample management and reproducibility:

Achieving precision starts with clear protocols. Use these best practices for sample management to ensure traceability, integrity, and efficiency in every experiment.
GEMINI (2025)
- Standardized naming conventions: Implement unique, traceable identifiers for all samples, reagents, and derived products. Barcoding and automated tracking systems significantly reduce the risk of human transcription errors.
- Controlled storage conditions: Ensure ultra-low temperature freezers and cryo-storage units are continuously monitored for temperature deviations. Critical samples should be stored in redundant systems to mitigate the risk of catastrophic loss.
- Comprehensive metadata: For every sample, record essential metadata, including donor information (appropriately de-identified), collection date, processing methods, passage number (for cell lines), and quality control results. High-quality metadata is critical for statistical power and later attempts at reproducibility.
The scientific community, including organizations like the National Institutes of Health (NIH), has increasingly emphasized research reproducibility as a core metric of quality. This push requires transparent reporting of methods and the precise use of characterized materials. Cell line authentication is required by major funding bodies, including the NIH, and strongly recommended as a best practice globally. By prioritizing rigorous documentation and centralized sample management, biological labs secure the foundation upon which high-impact discovery is built.
Implementing advanced imaging and technological integration
The pace of discovery in modern biological labs is often set by the capabilities of their instrumentation. Advanced imaging technologies—such as super-resolution microscopy, light-sheet microscopy, and high-throughput screening platforms—provide unprecedented views into complex biological systems, driving forward fields like cell biology and neuroscience. Successfully integrating these complex instruments requires specialized expertise and strategic planning.
Integration is not limited to microscopy. It extends to the complete digitalization of the experimental workflow, from robotic liquid handling to the final data analysis. In multidisciplinary labs, instrumentation cores serve as hubs of expertise, requiring dedicated technical staff to maintain and operate the machinery.
Considerations for advanced imaging and technology integration:
- Staff expertise: Advanced imaging systems require specialized training. Labs must invest in continuous education for technical staff, transitioning them from general technicians to specialized technologists capable of complex instrument calibration and troubleshooting.
- Data volume and storage: A single high-resolution image acquisition can produce terabytes of data. Biological labs must implement scalable data storage solutions and high-speed networking to handle the data deluge generated by these instruments, ensuring data integrity and accessibility for all relevant projects.
- Standardized protocols: To maximize the utility and reproducibility of results from shared resources, all users must adhere to strict, standardized protocols for instrument operation, sample preparation, and image analysis. This consistency is especially vital in multidisciplinary labs where diverse scientific goals share the same equipment.
- Open-source and proprietary software: Managing the complex software landscape, which includes proprietary control programs and open-source analysis packages (like ImageJ or Python-based tools), is a logistical challenge. Proper license management and version control are necessary to ensure that methods are robust and that analysis scripts can be run consistently over time.
Strategic investment in modern technology, coupled with the organizational infrastructure to support it, elevates a facility’s status. This capacity for cutting-edge analysis is essential for maintaining research relevance and attracting top talent, reinforcing the lab’s competitive edge in the scientific landscape.
From compliance to biosafety leadership: establishing a culture of safety
At the core of all laboratory operations lies the ethical and legal duty to maintain safety. Compliance with regulatory requirements, defined by agencies such as the Centers for Disease Control and Prevention (CDC) in the U.S. or the World Health Organization (WHO) internationally, establishes the minimum threshold for safety. However, true excellence in modern biological labs is characterized by the pursuit of biosafety leadership.
Biosafety leadership moves beyond checklists and mandates, advocating for a proactive, adaptive safety culture where every employee takes ownership of risk mitigation. This shift is crucial for facilities working across different biosafety levels (BSL-2, BSL-3, and the highly restricted BSL-4). The fundamental principles for handling infectious agents are outlined in documents like the Biosafety in Microbiological and Biomedical Laboratories (BMBL).
Key pillars of biosafety leadership:
Pillar | Focus Area | Impact on the Lab |
|---|---|---|
Risk assessment mastery | Moving beyond generic risk matrices to project-specific, dynamic risk assessments (DRA) that consider sample type, procedure, and personnel training level. | Reduces the likelihood of unforeseen exposure incidents; ensures protocols are tailored and effective. |
Culture of reporting | Establishing a non-punitive environment where near-misses and procedural deviations are immediately reported, analyzed, and used to improve SOPs. | Facilitates continuous improvement and fosters a transparent, ethical working environment. |
Continuous training | Ensuring all staff are rigorously trained for the specific biosafety levels in which they operate, including emergency response, spill management, and proper use of containment equipment. | Guarantees personnel competency and minimizes procedural errors, supporting regulatory compliance. |
Dedicated governance | Appointing a Biosafety Officer (BSO) with genuine authority and integrating the Institutional Biosafety Committee (IBC) into all project planning and review processes. | Elevates safety from an administrative task to a core scientific and ethical responsibility. |
Effective biosafety leadership requires consistent investment, both financially, as covered in the budget management section, and culturally. It ensures that the controls necessary for safe operation are treated with the same scientific rigor as the experiments themselves. This dedication not only protects the personnel but also the integrity of the research and the samples involved, directly supporting reproducibility and minimizing facility downtime due to safety breaches.
The implementation of enhanced practices in biosafety levels—such as strictly controlled access, mandatory use of primary containment equipment (e.g., biological safety cabinets), and rigorous waste management—must be constantly monitored. By institutionalizing these proactive measures, biological labs demonstrate a commitment to global health security and reinforce their role as trusted environments for scientific advancement.
Reinforcing scientific integrity through structured operations
The trajectory of modern biological labs is defined by the convergence of scientific ambition and operational rigor. Laboratory professionals are tasked with managing increasingly complex environments, from the strategic lab design that facilitates multidisciplinary labs to the financial prudence required for effective budget management. Above all, the commitment to scientific excellence must be guaranteed by high standards of reproducibility and meticulous sample management. This commitment must be secured by uncompromising adherence to safety protocols. A facility’s true measure of success is its capacity to deliver high-impact discoveries consistently while upholding the highest ethical and safety standards. This ultimate responsibility rests on embedding biosafety leadership—a cultural commitment that ensures the long-term protection of personnel, assets, and the public trust in scientific endeavor.
Frequently asked questions
How does biosafety leadership differ from standard biosafety compliance?
Standard biosafety compliance is the act of meeting the minimum legal and regulatory requirements, such as adhering to the guidelines for specific biosafety levels (BSL-2, BSL-3). Biosafety leadership, however, represents a proactive culture where personnel at all levels actively seek out and implement best practices that exceed the minimum required standard. This involves dynamic risk assessment, non-punitive reporting of near-misses, and continuous improvement of SOPs. It transforms safety from a regulatory burden into a core operational value, ensuring that all projects within biological labs are ethically grounded and systematically shielded from preventable hazards, thus securing data integrity and operational continuity. (149 words)
What is the most significant challenge in maintaining reproducibility across multidisciplinary labs?
The most significant challenge to reproducibility in multidisciplinary labs stems from the inherent variation in methodologies and instrumentation use across different research groups. When several teams share centralized equipment, such as advanced imaging systems or high-throughput sequencers, inconsistencies can arise from varied sample management techniques, different reagent sources, or subtle deviations in protocol execution. Overcoming this requires the implementation of a rigorous Laboratory Information Management System (LIMS) and mandatory, institution-wide SOPs for critical shared processes, coupled with transparent reporting of all experimental parameters to ensure findings can be accurately validated by other scientists. (150 words)
How can effective budget management support high biosafety levels in biological labs?
Effective budget management is foundational to operating facilities at high biosafety levels. These environments require substantial fixed costs, including specialized HVAC systems (like negative air pressure controls), robust redundant power supplies, and high-quality primary containment equipment (e.g., certified biological safety cabinets). Strategic budgeting ensures that funds are consistently allocated for preventative maintenance, mandatory certification of containment systems, and the procurement of necessary PPE. Cutting corners in these areas to save money directly compromises safety. Thus, a well-managed budget ensures the continuous operational readiness necessary for maintaining the stringent controls required for biosafety leadership within sensitive biological labs. (149 words)
What role does lab design play in achieving operational excellence and biosafety in modern facilities?
Lab design is instrumental in promoting operational excellence and biosafety leadership by defining the physical and functional architecture of the workspace. A well-designed facility incorporates designated zones for different activities, separating high-risk procedures from clean analysis areas to prevent cross-contamination. For biological labs, this also involves optimizing workflow to minimize unnecessary movement of personnel and samples, thereby reducing exposure risk and improving efficiency. Strategic placement of safety equipment, clear sightlines, and adequate space for advanced instrumentation (like advanced imaging devices) supports both adherence to biosafety levels and the overall reproducibility of experimental results. (149 words)
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.














