The transition from successful research and development protocols to high-volume manufacturing represents a significant challenge for industrial laboratories. Successful scale-up operations require physical infrastructure that anticipates future demand, maintains stringent quality control, and complies with evolving regulatory frameworks. Strategic foresight in designing industrial labs ensures that initial investments support long-term operational efficiency rather than becoming costly bottlenecks later in the lifecycle. This comprehensive analysis reviews the core principles necessary for creating resilient and adaptable laboratory environments capable of supporting accelerated industrial growth.
Modular Lab Design and Flexibility for Efficient Scale-Up
Flexibility represents the cornerstone of effective industrial lab design, directly correlating with a facility’s capacity for rapid scale-up. The physical layout must accommodate anticipated increases in personnel, equipment, and throughput without necessitating major structural renovations. A modular approach allows for the reconfiguration or expansion of spaces—such as moving or resizing processing zones—with minimal downtime and cost. This concept applies both to room arrangement and utility provision.
Design principles that support flexibility:
Design for Flexibility: Modern laboratories prioritize adaptability to meet evolving scientific needs.
GEMINI (2025)
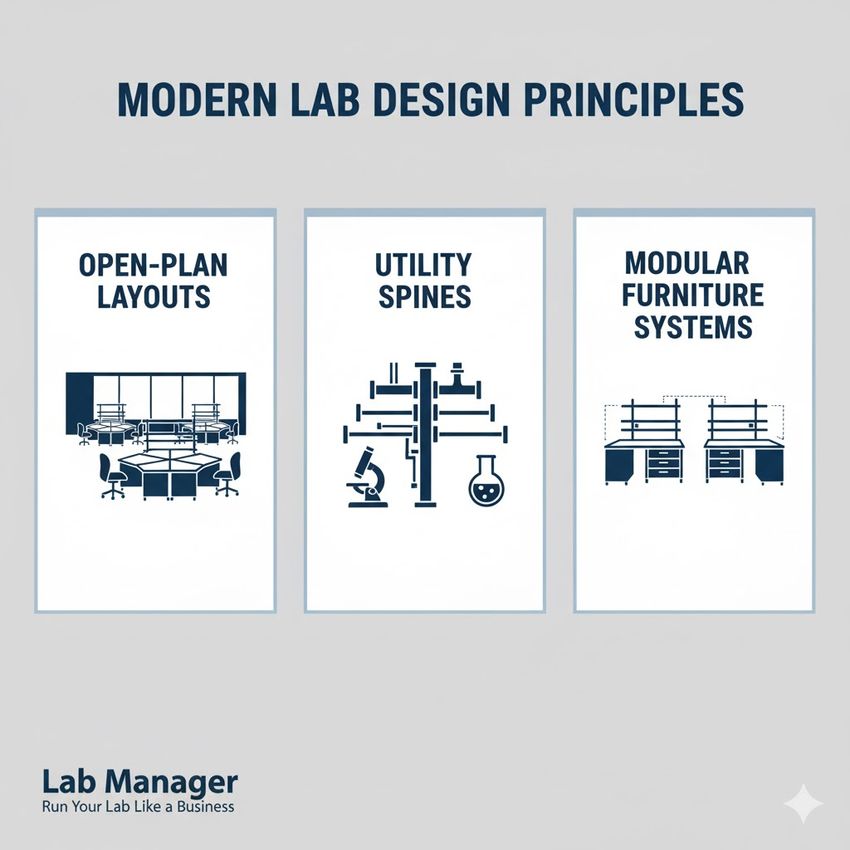
- Open-plan layouts: Utilizing large, column-free spaces facilitates the rearrangement of benches, biosafety cabinets, and robotic systems as processes change or expand.
- Utility spines: Centralized infrastructure pathways, often ceiling-mounted, deliver power, data, and gases through accessible connection points. This minimizes the need to break walls or ceilings when moving or adding new equipment.
- Modular furniture systems: Standardized, movable casework and benching allow for quick adjustments to workflow needs, supporting both initial setup and subsequent expansion stages.
The implementation of smart, modular lab design ensures that the operational environment remains agile. Furthermore, careful consideration of adjacency—placing high-throughput areas next to supporting quality control or warehousing functions—improves material flow and logistical efficiency during scale-up.
GxP Compliance and Safety Standards in Designing Industrial Labs
Regulatory compliance is non-negotiable when designing industrial labs, particularly those transitioning to GxP (Good Practice) environments (e.g., cGMP for pharmaceuticals). Facilities must implement design controls that guarantee product quality and data integrity from the outset. Early integration of compliance factors avoids costly retrofitting and validation efforts post-construction.
Safety considerations are equally vital and must exceed minimum regulatory standards. Comprehensive risk assessments inform the placement of specialized equipment, the design of ventilation systems, and the location of safety infrastructure. For instance, the process of designing industrial labs often involves creating distinct zones with controlled pressures and access to prevent cross-contamination, a requirement frequently audited by bodies such as the FDA.
Key compliance and safety integrations:
Category | Design Feature | Regulatory Relevance |
|---|---|---|
Airflow | Dedicated HVAC systems with HEPA filtration | ISO 14644 cleanroom standards; WHO Annex 1 provides related GMP guidance. |
Surfaces | Non-porous, smooth, and easily cleanable materials | Contamination control, sterilization protocols |
Documentation | Dedicated data infrastructure and controlled access points | 21 CFR part 11 (Data integrity and electronic records) |
Containment | Fume hoods, biosafety cabinets, secondary containment | OSHA (Occupational Safety and Health Administration) guidelines for chemical/biological hazards |
The commitment to compliance informs every aspect of lab design, safeguarding personnel and validating process integrity, thereby enabling smooth regulatory approval necessary for commercial scale-up.
Infrastructure Planning for Utility Demand in Industrial Lab Scale-Up
High-throughput industrial operations place immense and continuous demands on utilities, making robust infrastructure planning critical when designing industrial labs. Utility systems, including electrical power, purified water, specialized gases, and waste streams, must be sized not only for the initial capacity but for the maximum projected future capacity. Undersized utility infrastructure represents one of the most common and expensive roadblocks to effective scale-up.
Advanced Lab Management Certificate
The Advanced Lab Management certificate is more than training—it’s a professional advantage.
Gain critical skills and IACET-approved CEUs that make a measurable difference.
Adequate space must be allocated in utility corridors, basements, or mezzanines for large equipment such as air handlers, water purification units, and electrical substations. Furthermore, incorporating redundancy—such as backup generators, uninterruptible power supplies (UPS), and dual-feed water loops—is essential to mitigate the risk of catastrophic failures and maintain operational continuity. A reliable infrastructure foundation allows the laboratory to increase operational hours and equipment count without risking service interruptions.
Critical utility considerations:
- Electrical load: Calculation of total demand, including future automation and cryogenic storage units, requiring dedicated circuits and proper grounding.
- Water quality: Selection of appropriate water purification systems (RO, DI, WFI) with sufficient flow rates and storage capacity for peak usage.
- Waste management: Design of segregated waste streams for chemical, biological, and general waste, adhering to environmental protection agency (EPA) guidelines
- Environmental control: Installation of monitoring systems for temperature, humidity, and particulate matter to ensure stability across all operational zones.
Integrating Automation and Optimizing Material Flow for Scale-Up
The operational efficiency required for scale-up hinges on the successful integration of automated systems and optimized material flow. The layout of the laboratory should minimize human movement, reduce manual handling errors, and maximize the operational footprint of robotic equipment.
When designing industrial labs, a strategic approach places automated work cells in linear or parallel arrangements that mirror the sequential steps of the manufacturing process. This reduces the distance and complexity of moving samples, reagents, or products between stations. Furthermore, ergonomic considerations in the placement of control stations and input/output zones for automated equipment are paramount for operator comfort and safety.
Optimized material flow entails mapping out every path a material takes, from raw material receipt to final product shipment. This includes dedicated lanes for clean materials and finished goods, separation from waste pathways, and designated staging areas. The physical design—including corridor widths, door types, and flooring—must support the safe and efficient movement of heavy or sensitive equipment. Proper material management planning directly enhances throughput and minimizes the contamination risk associated with the high volume expected during scale-up.
Conclusion: A Strategic Approach to Designing Industrial Labs
Effective designing industrial labs requires an integrated approach that views the physical facility as a critical component of the manufacturing process. By prioritizing modularity, anticipating GxP compliance needs, investing in robust and redundant utility infrastructure, and integrating automation for streamlined workflow, laboratory professionals can create environments primed for efficient and rapid scale-up. Strategic planning in these core areas converts capital expenditure into a sustainable platform for long-term operational success.
Frequently asked questions
What is the primary objective when designing industrial labs?
The primary objective involves creating a highly efficient, scalable, and compliant environment that can transition smoothly from small-scale R&D processes to high-volume production without major structural modifications.
How does modular lab design aid scale-up?
Modular lab design utilizes standardized components and flexible utility systems, allowing for quick, low-cost reconfiguration of workspaces and addition of new equipment to meet increased throughput demands, thus facilitating easier scale-up.
Which regulatory body's guidelines most influence industrial lab design?
For pharmaceutical and biotechnology scale-up, the FDA (Food and Drug Administration) and corresponding international bodies heavily influence lab design through GxP requirements, particularly concerning contamination control, data integrity, and environmental monitoring.
What is the biggest infrastructure bottleneck during rapid scale-up?
The most common bottleneck involves undersized or non-redundant utility systems, such as electrical supply, HVAC capacity, or water purification, which cannot support the increased operational load and equipment count required for full scale-up.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.












