The operational core of the clinical laboratory centers on generating accurate, timely results that directly impact patient care decisions. Increased testing volumes, complex assay menus, and the constant demand for faster turnaround times require strategic enhancements to workflow and data handling. Adopting sophisticated diagnostic platforms and deploying comprehensive automation systems represent essential innovations for navigating these challenges. These systems transform high-volume testing into a streamlined, high-quality operation. Maintaining a competitive edge and meeting stringent regulatory demands depends on the thoughtful integration of these modern technologies into existing laboratory infrastructures.
The evolution of diagnostic platforms and testing efficiency
The sophistication of modern diagnostic platforms fundamentally changes the way laboratories approach patient testing, driving both efficiency and analytical reliability. These highly specialized systems, known as in vitro diagnostics (IVD), encompass analytical instruments, reagents, and associated software necessary for performing specific assays. The current generation of platforms offers unparalleled sensitivity and specificity, often integrating multiple steps of the analytical process into a single, closed system. This integration minimizes manual handling steps, thereby reducing the potential for human error and sample contamination.
Key features driving these innovations include miniaturization and multiplexing capabilities. Miniaturization enables the use of smaller sample volumes, which benefits pediatric and critical care settings where sample availability is limited. Multiplexing allows simultaneous testing for numerous analytes from a single sample, dramatically improving diagnostic yield and conserving precious resources. The World Health Organization (WHO) emphasizes that robust IVD technologies are critical components of resilient health systems, particularly when managing infectious disease outbreaks or complex chronic conditions. Furthermore, regulatory bodies, such as the US Food Drug Administration (FDA), maintain strict oversight to ensure these commercial diagnostic platforms meet rigorous performance standards before clinical implementation. Clearance often requires pathways such as the 510(k) premarket notification or the Premarket Approval (PMA).
High-throughput systems:
- Consolidated Testing: Combining multiple assay types (e.g., chemistry, immunoassay) onto a single, automated analyzer.
- Random Access: Allowing samples to be loaded and analyzed continuously, regardless of the test requested, optimizing analyzer utilization.
- Standardized Interfaces: Simplifying the connection of the platform to broader laboratory automation tracks or conveyor systems.
Laboratory information management systems (LIMS) as the central nervous system
A laboratory information management system, or LIMS, provides the critical infrastructure necessary for managing the vast amounts of data generated by modern diagnostic platforms and sophisticated automation systems. The LIMS functions as the central nervous system of the laboratory, overseeing the entire sample lifecycle from accessioning to result reporting. Proper LIMS integration is essential for ensuring data integrity, traceability, and regulatory compliance.
The system ensures that every step of the testing process is documented and auditable, which is vital for accreditation compliance. It interfaces directly with analytical instruments to capture results automatically, eliminating manual transcription errors. Effective LIMS implementation also facilitates resource planning, inventory control, and staff management by providing real-time operational data. Industry standards, such as those published by the Clinical and Laboratory Standards Institute (CLSI), provide extensive guidelines for laboratory data management practices. These guidelines emphasize data security and integrity within the LIMS framework.
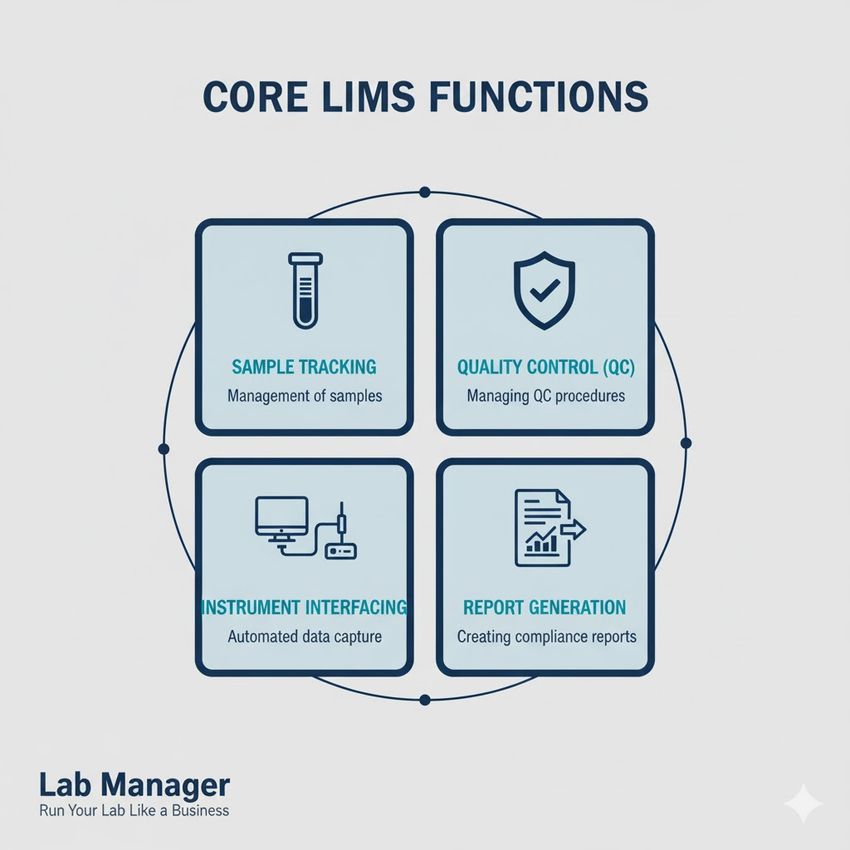
Core LIMS functionalities include:
Your LIMS system is the core engine for efficiency and compliance.
GEMINI (2025)
- Sample Tracking: Assigning unique identifiers and monitoring the sample’s location and status throughout the laboratory.
- Quality Control (QC) Management: Automatically flagging QC failures and preventing the release of patient results until corrective action is documented.
- Instrument Interfacing: Bi-directional communication with diagnostic platforms to send test orders and receive results.
- Report Generation: Producing standardized, validated patient reports ready for clinical review.
Transforming throughput with total laboratory automation (TLA)
Total laboratory automation (TLA) represents a comprehensive strategy for integrating all phases of the testing process—pre-analytical, analytical, and post-analytical—using robotic systems and sophisticated conveyor tracks. This level of automation delivers substantial gains in efficiency, standardization, and personnel safety, making it a critical aspect of modern high-volume clinical labs. TLA connects various specialized diagnostic platforms, such as hematology, chemistry, and immunoassay analyzers, into a seamless, continuous workflow.
In the pre-analytical phase, TLA handles sample sorting, decapping, centrifugation, and aliquoting, which are historically the most error-prone manual steps. During the analytical phase, samples move autonomously to the appropriate analyzers, which are managed by the LIMS. The post-analytical phase includes automated sample recapping, storage, and retrieval, enabling efficient add-on testing without manual searching. A dedicated focus on TLA implementation, as documented in peer-reviewed laboratory management journals, demonstrates significant reductions in laboratory errors and improvements in turnaround time by up to 30-50%. The use of advanced robotics also mitigates ergonomic hazards, supporting compliance with workplace safety guidelines established by organizations like the Occupational Safety and Health Administration (OSHA).
Emerging innovations in data analytics and artificial intelligence
The continuous drive for innovations extends beyond hardware and into the realm of computational intelligence, where data analytics and artificial intelligence (AI) are unlocking new levels of operational efficiency. Machine learning algorithms, when applied to the vast data streams originating from diagnostic platforms and LIMS, can identify subtle patterns that human analysts might overlook. This includes predictive maintenance, where machine-learning models can predict instrument failure by analyzing operational metrics, allowing for proactive servicing and minimizing downtime.
AI also significantly improves internal quality control (QC). Algorithms can analyze QC results across multiple testing runs, detecting small drifts or shifts earlier than traditional statistical methods. This proactive QC intervention maintains high analytical reliability and reduces the risk of releasing erroneous patient results. Furthermore, AI-driven decision support tools assist pathologists and clinical scientists by prioritizing complex cases, flagging potential critical values, and providing contextual information from integrated patient histories. This ensures timely and informed clinical actions. These computational innovations maximize the utility of the laboratory data archive, transforming it from a mere repository into an active tool for continuous improvement and high-quality patient outcomes.
Data integrity and cybersecurity challenges in the automated laboratory
The profound connectivity inherent in LIMS and advanced automation systems introduces critical requirements for maintaining data integrity and robust cybersecurity protocols. Digital records must remain secure, confidential, and unaltered from the point of generation on the diagnostic platforms through to final archival. Unauthorized access, modification, or destruction of patient data poses severe legal and ethical risks, particularly concerning Protected Health Information (PHI). Preventing such breaches necessitates multi-layered security measures, including comprehensive encryption, stringent access controls, and regular system audits. Secure network segmentation ensures that automation tracks and analytical instruments operate on protected subnets, minimizing exposure to external threats. Furthermore, establishing redundant data backups and disaster recovery plans guarantees operational continuity following any system compromise or failure. Laboratory management holds the responsibility for implementing a proactive cybersecurity posture, aligning with regulatory frameworks to safeguard the sensitive information entrusted to the laboratory’s digital systems.
Future operational strategies: maximizing throughput with automation and diagnostic platforms
The modern laboratory benefits significantly from the synergistic integration of advanced diagnostic platforms, comprehensive LIMS, and sophisticated automation solutions. Continued refinement of these technologies promises even greater efficiency gains, ensuring the laboratory can handle increasing volumes while maintaining impeccable quality. Strategic investment in analytical innovations and integrated informatics systems represents the clearest path toward achieving optimal throughput and supporting the highest standards of patient care. The future demands highly adaptable, interconnected laboratory systems capable of rapid deployment and standardization across diverse testing environments.
Frequently asked questions
How do diagnostic platforms ensure data accuracy?
Diagnostic platforms ensure data accuracy through built-in quality control mechanisms, automated calibration routines, and strict regulatory validation before commercial release. Automated result transmission to the LIMS eliminates transcription errors, further safeguarding the integrity of patient data.
What is the primary benefit of total laboratory automation (TLA)?
The primary benefit of total laboratory automation is the standardization and acceleration of the testing process, particularly the error-prone pre-analytical and post-analytical steps. This leads to faster turnaround times, increased staff safety, and reduced overall laboratory errors.
What role does the LIMS play in adopting new innovations?
The LIMS serves as the central hub for integrating new innovations. It provides the interface for new diagnostic platforms to connect with existing workflows, ensuring data compatibility, maintaining traceability, and managing quality control procedures for all new assays introduced.
How does automation improve laboratory personnel safety?
Automation significantly improves laboratory personnel safety by minimizing manual handling of biohazardous samples, reducing repetitive strain injuries associated with manual processing tasks, and decreasing staff exposure to chemical reagents.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.














