In the fast-paced world of modern laboratories, the demand for fast, sensitive, and cost-effective analytical methods is constant. While a myriad of techniques exist, one discipline stands out for its versatility and precision: electrochemical analysis. At its core, this field of analytical chemistry involves the measurement of electrical properties—such as voltage, current, or resistance—to gain insights into the chemical properties of a solution. From measuring pH in a bioreactor to detecting heavy metals in an environmental sample, electrochemical methods have become indispensable tools in clinical diagnostics, pharmaceutical development, environmental monitoring, and materials science.
For lab professionals, a solid understanding of these techniques is crucial. They offer distinct advantages, including excellent sensitivity for trace-level analysis, a wide linear dynamic range, and a relatively low cost of instrumentation. Furthermore, many electrochemical methods are well-suited for automation and miniaturization, making them ideal for high-throughput screening and point-of-care diagnostics. This article will provide a detailed overview of the foundational and advanced techniques within electrochemical analysis, empowering you to leverage these powerful tools for enhanced analytical precision in your laboratory.
The Foundation: Understanding the Principles of Electrochemical Analysis
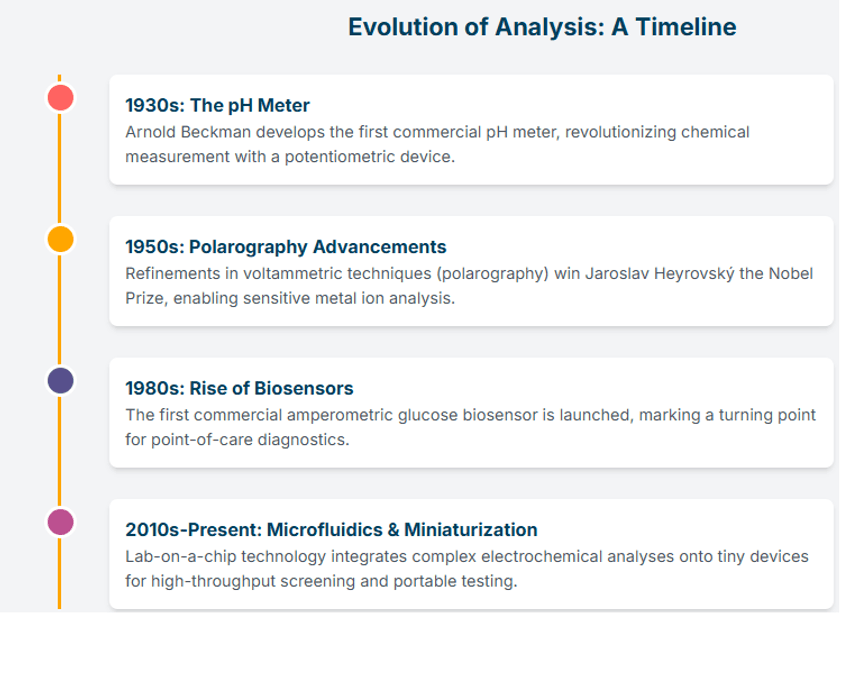
Electrochemical analysis developments over time.
GEMINI (2025)
At the heart of every electrochemical measurement is a chemical reaction involving the transfer of electrons, known as a redox reaction. These reactions can be driven by an external electrical potential or can themselves generate a potential. Electrochemical techniques are broadly categorized based on the electrical property being measured and how it is controlled.
The fundamental setup for most quantitative electrochemical analysis involves an electrochemical cell, which typically contains three key components:
- Working Electrode (WE): This is where the redox reaction of interest occurs. The potential of this electrode is precisely controlled relative to a reference electrode.
- Reference Electrode (RE): This electrode provides a stable and known potential against which the working electrode's potential is measured or controlled. It is crucial for maintaining a constant baseline. Common examples include the saturated calomel electrode (SCE) and the silver/silver chloride (Ag/AgCl) electrode.
- Counter Electrode (CE): This electrode completes the circuit. It carries the current needed to balance the current flowing at the working electrode, ensuring that the potential of the working electrode is not influenced by the current passing through the reference electrode.
The relationship between the chemical and electrical properties is governed by several fundamental principles, including:
- Faraday's Laws of Electrolysis: These laws relate the amount of substance produced or consumed at an electrode to the quantity of electrical charge passed through the cell. This principle is foundational to coulometry.
- The Nernst Equation: This equation describes the relationship between the potential of an electrode and the concentration of the species undergoing a redox reaction. It is the cornerstone of potentiometric measurements.
Understanding these principles is the first step toward mastering the diverse techniques that fall under the umbrella of electrochemical analysis.
Potentiometry: A Cornerstone of pH and Ion Measurement
Potentiometry is arguably the simplest and most widely used form of electrochemical analysis. It is a zero-current technique, meaning it measures the potential difference between two electrodes when no net current is flowing through the cell. This potential is a direct function of the concentration or activity of a specific ion in the solution, as described by the Nernst equation.
The most common application of potentiometry is the measurement of pH using a glass electrode. The potential of the glass electrode changes in response to the activity of hydrogen ions (H+) in the solution. Beyond pH, potentiometry is invaluable for:
- Ion-Selective Electrodes (ISEs): These specialized electrodes are designed to respond selectively to a single type of ion. They are used to measure a wide range of ions, including sodium (Na+), potassium (K+), calcium (Ca2+), fluoride (F−), and chloride (Cl−). ISEs are crucial in clinical labs for electrolyte analysis and in environmental monitoring for water quality assessment.
- Potentiometric Titrations: In this method, a titrant is added to a solution, and the potential of an indicator electrode is monitored. The endpoint of the titration is determined by a sharp change in potential, eliminating the need for a visual indicator and providing greater accuracy.
Method | Principle | Key Application |
|---|---|---|
Potentiometry | Measures potential difference at zero current. | pH measurement, Ion-selective electrodes for Na+, K+, F−. |
Voltammetry | Measures current as a function of applied potential. | Trace metal analysis, drug quantification, reaction mechanism studies. |
Coulometry | Measures the total charge passed during a reaction. | Karl Fischer titration for water content, quantitative analysis of pure substances. |
Amperometry | Measures current at a constant potential. | Glucose biosensors, chlorine detection in water. |
Voltammetry: Unlocking Quantitative and Qualitative Analysis
Unlike potentiometry, voltammetry is a dynamic technique that measures the current passing through an electrochemical cell as a function of the applied potential. By systematically sweeping or pulsing the potential of the working electrode, a characteristic plot called a voltammogram is generated. This plot provides a wealth of information about the analyte, including its identity (qualitative analysis) and concentration (quantitative analysis).
There are several types of voltammetry, each with its own advantages:
- Cyclic Voltammetry (CV): In CV, the potential is scanned in a forward and reverse direction, creating a current-potential curve that resembles a butterfly-like shape. It is a powerful tool for studying the kinetics and mechanisms of redox reactions, as it can reveal information about reaction reversibility, electron transfer rates, and the presence of intermediate species.
- Differential Pulse Voltammetry (DPV) and Square Wave Voltammetry (SWV): These are pulsed techniques that apply small, successive potential pulses to the working electrode. They are significantly more sensitive than classical voltammetry and are widely used for trace analysis of organic compounds, pharmaceuticals, and heavy metals. The pulsed nature of these methods helps to minimize background current, leading to a much better signal-to-noise ratio.
Voltammetry’s ability to provide both qualitative and quantitative data makes it a preferred method for a wide range of applications, from quantifying lead in drinking water to analyzing the concentration of a new drug compound.
Beyond the Basics: Coulometry and Other Advanced Techniques
While potentiometry and voltammetry are the workhorses of electrochemical analysis, other specialized techniques offer unique advantages for specific applications.
- Coulometry: This method is based on Faraday's laws of electrolysis. It measures the total amount of charge (in Coulombs) required to completely oxidize or reduce an analyte in a solution. Coulometry is an absolute method, meaning it doesn’t require calibration standards and is highly accurate. A key application is Karl Fischer titration, which uses coulometry to precisely determine the water content in a sample, a critical measurement in the pharmaceutical and petrochemical industries.
- Amperometry: This technique measures the current at a constant applied potential. It is often used in a detection mode, for example, in chromatography to detect electroactive compounds as they elute from a column. The most famous example of amperometry in action is the modern glucose biosensor, which measures the current produced by the oxidation of glucose to determine blood sugar levels.
- Conductometry: This method measures the electrical conductivity of a solution, which is proportional to the concentration and mobility of the ions present. It is particularly useful for monitoring total dissolved solids in water and for conductometric titrations where the change in conductivity is monitored as a titrant is added.
The true power of electrochemical analysis lies in the complementary nature of these methods. By combining different techniques, lab professionals can obtain a more complete picture of a sample's chemical composition and behavior.
The Future of Electrochemical Analysis in Your Lab
Electrochemical methods have evolved from simple pH meters to sophisticated, microfluidic devices capable of high-throughput analysis. Their inherent advantages—high sensitivity, rapid response, and a low cost of instrumentation—ensure their continued relevance and growth. Miniaturization has enabled the creation of portable and handheld devices for on-site testing, while advancements in electrode materials and surface modification continue to push the limits of sensitivity and selectivity.
For lab professionals, embracing these techniques is an investment in the future of analytical chemistry. By understanding the principles of potentiometry, voltammetry, and other related methods, you can select the right tool for the job, optimize your analytical workflows, and deliver accurate, reliable results that meet the demands of your industry.
Frequently Asked Questions about Electrochemical Analysis
What is the key difference between potentiometry and voltammetry?
Potentiometry measures a potential difference at zero current to determine analyte concentration, while voltammetry measures the current generated as a function of a controlled, changing potential to get both qualitative and quantitative data.
Why is potentiometry a cornerstone for pH and ion measurements?
Potentiometry is an ideal technique for these measurements because it is a non-destructive method that directly relates the measured potential to the concentration of the target ion or species, as described by the Nernst equation. This provides a simple, fast, and accurate way to determine pH or ion activity.
What are the primary advantages of using electrochemical analysis over other methods?
The main advantages include high sensitivity, a wide linear dynamic range, minimal sample preparation, rapid analysis times, and cost-effective instrumentation. Many electrochemical methods are also well-suited for automation and portable applications.
How does a three-electrode system improve analytical results?
A three-electrode system (working, reference, and counter) provides precise control over the potential of the working electrode. This separation of function ensures that the current flowing at the working electrode does not affect the stable potential of the reference electrode, leading to more accurate and reliable measurements.
















