In the complex and rapidly evolving landscape of modern science, from pharmaceutical discovery to food safety and environmental protection, a single, indispensable discipline underpins every critical decision: analytical chemistry. It is the silent workhorse of the laboratory, providing the quantitative and qualitative data that validates research, ensures product quality, and safeguards public health. This article explores the core principles, essential techniques, and future trends of analytical chemistry, offering a comprehensive guide for laboratory managers, quality professionals, and scientists navigating the demands of a data-driven world.
Principles of Analytical Chemistry: A Core Definition
At its core, analytical chemistry is the science of measurement. It is a field dedicated to the characterization of matter, answering two fundamental questions about a sample: “What is it?” (qualitative analysis) and “How much of it is there?” (quantitative analysis). It involves a diverse array of methods and instruments used to separate, identify, and quantify the components of a sample.
This discipline is not merely a collection of techniques; it is a systematic approach to problem-solving. It requires a deep understanding of chemical principles to select the appropriate method, prepare the sample correctly, operate sophisticated instrumentation, and, most critically, interpret the results accurately. It is a foundational pillar for virtually every scientific field, from clinical diagnostics and materials science to forensics and archaeology. Without the precision of analytical chemistry, our understanding of the world at the molecular level would be speculative at best.
Practical Applications of Analytical Chemistry: Why It's Essential
The significance of analytical chemistry extends far beyond the confines of the laboratory, directly impacting safety, commerce, and innovation. Its practical applications are woven into the fabric of modern life, ensuring the quality and integrity of the products and environment we interact with daily.
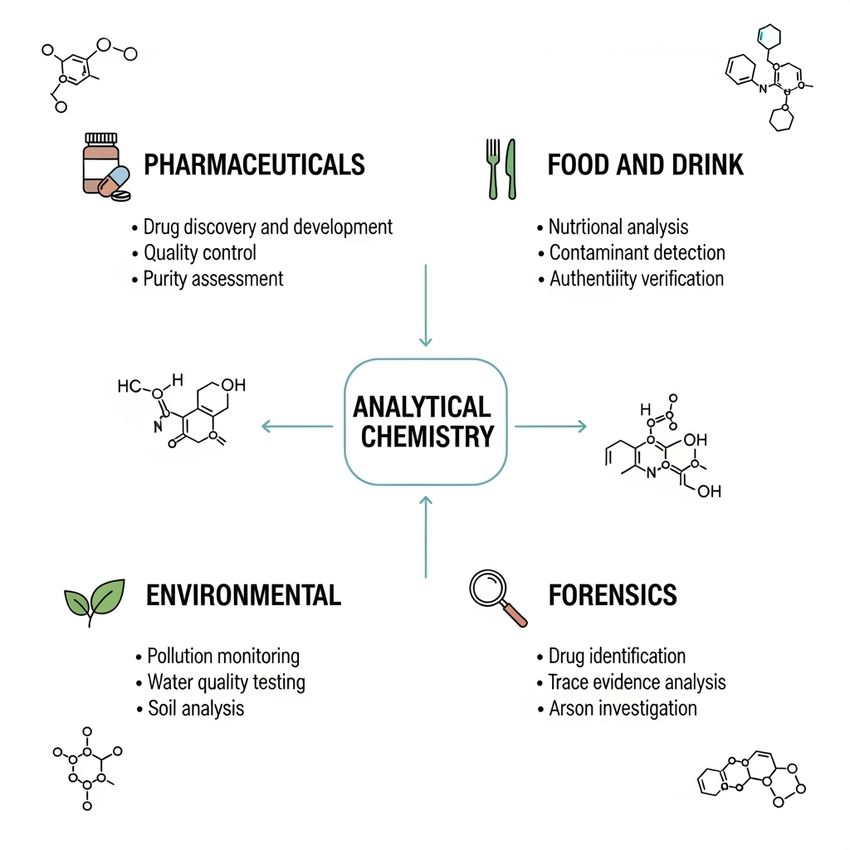
The significance of analytical chemistry extends far beyond the confines of the laboratory. GEMINI (2025)
- Pharmaceuticals and Life Sciences: In the drug development lifecycle, analytical chemistry is paramount. It is used to identify and characterize new drug compounds, monitor the stability and purity of active pharmaceutical ingredients (APIs), and ensure that every tablet or vial contains the correct dose. Techniques like High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) are essential for validating the identity and concentration of a drug substance, ensuring patient safety and regulatory compliance.
- Food and Beverage Industry: From farm to table, analytical chemistry guarantees the safety and quality of our food supply. It is used to detect contaminants like pesticides, heavy metals, and mycotoxins, ensuring products meet safety standards. It also helps to verify nutritional information, such as sugar, fat, and vitamin content, and to authenticate products, for example, by detecting adulteration in olive oil or honey.
- Environmental Monitoring: Analytical chemists are on the front lines of protecting our planet. They use advanced techniques to test water, soil, and air samples for pollutants. By quantifying trace amounts of contaminants like industrial chemicals or microplastics, they provide the critical data needed to enforce environmental regulations, track pollution sources, and assess ecological health.
- Forensic Science: The courtroom often relies on the evidence provided by analytical chemistry. From identifying unknown substances at a crime scene to DNA analysis and toxicology, these techniques provide objective data to support criminal investigations. Techniques like Gas Chromatography-Mass Spectrometry (GC-MS) are routinely used to analyze trace evidence or screen for illicit drugs in bodily fluids.
The Analytical Chemistry Process: A Step-by-Step Guide
A successful analytical chemistry workflow is a meticulous, multi-step process that demands precision at every stage.
Problem Definition and Method Selection: This is the most crucial step. The analyst must clearly define the objective: What substance needs to be measured? At what concentration? With what level of accuracy? The answers guide the selection of the most appropriate analytical technique and instrumentation.
Sampling: The sample must be representative of the whole. Improper sampling can lead to biased results, regardless of how accurate the subsequent analysis is. This step involves collecting, labeling, and preserving the sample to prevent degradation.
Sample Preparation: Raw samples are rarely suitable for direct analysis. This step involves a series of procedures to prepare the sample for introduction into the instrument. Common techniques include dissolution, extraction, filtration, or derivatization to make the analyte more volatile or easier to detect.
Analysis: The prepared sample is introduced into the analytical instrument. The instrument separates the components (e.g., using chromatography) and then detects and quantifies the analyte of interest (e.g., using a spectrometer or mass analyzer).
Data Analysis and Interpretation: The raw data from the instrument is processed and interpreted. This involves calibrating the instrument using standards, performing calculations, and using software to generate results. The data must be checked for anomalies and trends.
Reporting and Documentation: The final results are documented in a clear and concise report, complete with all relevant information, including the method used, calibration data, and any limitations. Proper documentation is essential for traceability and compliance.
Ensuring Quality in Analytical Chemistry: Key Performance Parameters
To ensure the reliability of analytical data, methods are evaluated against several key performance parameters. These metrics are the foundation of method validation and are required by regulatory bodies.
- Accuracy: This refers to the closeness of a measured value to the true or accepted value. An accurate method produces results that are free from systematic errors or bias. For example, if a standard sample is known to contain 100 ppm of a compound, an accurate analytical method should consistently yield a result very close to that value.
- Precision: This is a measure of the reproducibility or repeatability of a method. It reflects how close multiple measurements are to each other under the same conditions. A precise method will produce a tight cluster of results, even if they are not entirely accurate. Precision is often expressed as the standard deviation or relative standard deviation (RSD) of a set of measurements.
- Specificity and Selectivity: Specificity is the ability of a method to measure a single, target analyte without interference from other components in the sample matrix. Selectivity is the ability to distinguish the analyte from other components. For instance, in a complex biological sample, a specific method for a drug molecule must be able to ignore background proteins, salts, and endogenous compounds.
- Limit of Detection (LOD) and Limit of Quantitation (LOQ): The LOD is the lowest concentration of an analyte that can be reliably detected, but not necessarily quantified. The LOQ is the lowest concentration that can be reliably quantified with an acceptable level of accuracy and precision. These parameters are crucial for trace analysis, such as testing for residual solvents in a pharmaceutical product.
- Linearity and Range: Linearity describes the ability of a method to produce results that are directly proportional to the concentration of the analyte within a specific range. The range is the interval over which the method is linear, accurate, and precise. A method's linear range defines the concentrations for which it is suitable.
- Robustness: This is a measure of a method's capacity to remain unaffected by small, deliberate variations in method parameters, such as changes in column temperature or mobile phase composition. A robust method is less likely to produce inconsistent results when implemented by different analysts or in different laboratories.
Navigating Regulations: Analytical Chemistry and Compliance
Compliance with regulatory guidelines is a non-negotiable aspect of modern analytical chemistry, especially in regulated industries like pharmaceuticals and food production. These guidelines ensure data integrity, method reliability, and product safety.
- FDA (Food and Drug Administration): The FDA’s Current Good Manufacturing Practices (cGMP) regulations are the cornerstone for pharmaceutical quality control. They mandate that all analytical methods used for product release and stability testing are fully validated and documented. Data integrity is a major focus, requiring electronic records to be secure, traceable, and attributable to the person who generated them (e.g., 21 CFR Part 11).
- ICH (International Council for Harmonisation): The ICH provides globally harmonized guidelines for pharmaceutical development. The ICH Q2(R1) guideline, "Validation of Analytical Procedures," is a critical document that defines the validation parameters and requirements for various analytical methods, including identification, impurity testing, and assay procedures. Adherence to ICH guidelines ensures that analytical data is accepted by multiple regulatory bodies worldwide.
- ISO (International Organization for Standardization): ISO standards, particularly ISO/IEC 17025, provide a framework for the competence of testing and calibration laboratories. Achieving ISO 17025 accreditation demonstrates that a laboratory operates a robust quality management system and is technically competent to produce valid results. This is especially important for environmental and clinical testing labs.
Overcoming Challenges in Modern Analytical Labs
Despite technological advancements, managing a modern analytical laboratory comes with a unique set of challenges. Proactively addressing these issues is critical for maintaining efficiency and data quality.
- Data Integrity and Security: One of the most significant challenges is ensuring the integrity and security of analytical data. This includes preventing data manipulation, ensuring all data is saved and backed up, and maintaining a complete audit trail. The causes of data integrity issues often stem from inadequate Standard Operating Procedures (SOPs), insufficient training, or a lack of proper LIMS (Laboratory Information Management System) software that enforces data security.
- Method Validation Complexity: The process of validating a new analytical method is notoriously time-consuming and labor-intensive. It requires extensive testing to prove that the method is fit for its intended purpose and meets all regulatory requirements. The root cause is the sheer number of parameters (accuracy, precision, linearity, etc.) that must be tested and documented, often for a wide range of sample matrices.
- Instrument Downtime and Maintenance: High-end analytical instruments like Mass Spectrometers and GC-MS systems are complex and require regular maintenance. Unexpected instrument downtime can halt projects, delay product releases, and cause significant financial losses. This is often caused by a lack of proactive preventive maintenance, insufficient inventory of spare parts, or a shortage of trained service personnel.
- Sample Matrix Effects: The presence of other compounds in a sample can interfere with the analysis of the target analyte, leading to inaccurate results. For example, in a complex food sample, sugars or fats might suppress the signal of a pesticide during mass spectrometry. Mitigating this challenge requires extensive sample preparation and the development of highly specific methods, which can be time-consuming.
- Staff Training and Expertise: Operating advanced analytical equipment requires specialized knowledge and continuous training. A lack of trained personnel can lead to a high rate of errors, compromised data quality, and reduced lab productivity. The causes include the rapid evolution of technology and the high cost of specialized training courses.
Optimizing Your Lab: Best Practices for Analytical Chemistry Managers
Successful lab managers do more than just oversee operations; they create an environment of continuous improvement and quality.
- Invest in Continuous Training and Skill Development: The field of analytical chemistry is constantly advancing. Managers should prioritize and budget for ongoing training for their staff. This includes not only training on new instruments and software but also professional development in data integrity, quality assurance, and regulatory compliance.
- Embrace Automation and Digitalization: Implementing robotic sample handlers, automated solid-phase extraction (SPE) systems, and liquid handling platforms can significantly reduce manual errors and increase sample throughput. A robust LIMS can streamline workflows, manage sample data, and ensure data integrity, while integrating instruments directly to the LIMS can eliminate transcription errors.
- Implement a Robust Quality Management System (QMS): A well-defined QMS is the backbone of a high-quality analytical lab. It includes documented procedures (SOPs), regular internal audits, and a system for corrective and preventive actions (CAPA). A strong QMS ensures that every aspect of the laboratory operation is controlled, documented, and reproducible.
- Prioritize Method Validation and Verification: Do not view method validation as a mere regulatory checklist. A thoroughly validated method is a reliable method. For every new method, managers should ensure that the validation is scientifically sound and comprehensive. For methods transferred from a different lab or validated elsewhere, a verification process is essential to ensure it performs as expected in your specific laboratory environment.
- Foster a Culture of Data Integrity and Accountability: Data integrity is not just a technological issue; it's a cultural one. Lab managers should promote a culture where accurate and honest data is valued above all else. This includes providing clear guidance on data handling, transparently documenting all deviations, and holding all staff accountable for data quality.
The Future of Analytical Chemistry: Emerging Lab Trends
Analytical chemistry is a dynamic and essential science that serves as the quality assurance arm of countless industries. From the meticulous analysis of drug compounds to the detection of environmental pollutants, it provides the critical, objective data needed to make informed decisions.
The field is poised for a new era of innovation. The future of analytical chemistry is being shaped by several exciting trends:
- Miniaturization and Portability: The development of smaller, more portable instruments will allow for on-site analysis, moving the lab from a centralized location to the point of need—whether that's a remote environmental site, a production floor, or a patient’s bedside.
- Artificial Intelligence and Machine Learning: AI is set to revolutionize data analysis by automating spectral interpretation, predicting sample properties, and optimizing instrument parameters. Machine learning algorithms will be able to find subtle patterns in complex datasets, leading to faster and more accurate diagnoses and discoveries.
- High-Resolution Mass Spectrometry (HRMS): HRMS is offering unprecedented levels of accuracy and sensitivity, enabling the identification and quantification of thousands of compounds in a single sample. This technology is driving advancements in metabolomics, proteomics, and exposomics, providing a holistic view of biological systems and environmental exposures.
- Single-Cell Analysis: Pushing the limits of sensitivity, new analytical techniques are enabling the characterization of individual cells. This is a game-changer for medical research, as it can reveal biological differences between cells in a heterogeneous population, leading to new insights into diseases like cancer.
- Hyphenated Techniques: The combination of multiple analytical techniques in a single instrument, known as "hyphenation" (e.g., GC-MS/MS or LC-ICP-MS), will continue to be a powerful trend, providing analysts with more comprehensive and reliable data from a single run.
By embracing these trends and adhering to sound principles and best practices, modern laboratories can continue to be a source of trust, innovation, and scientific excellence.
FAQs about Analytical Chemistry
1. What is the primary role of analytical chemistry in quality control? The primary role of analytical chemistry is to provide accurate and reliable data that confirms the identity, purity, and concentration of a substance. This is crucial for quality control (QC), as it ensures that raw materials meet specifications, products are consistent, and final goods are safe for consumer use, all in compliance with regulatory standards.
2. What are the most common techniques in modern analytical chemistry? Common analytical chemistry techniques include chromatography (e.g., Gas Chromatography or GC, High-Performance Liquid Chromatography or HPLC) for separating mixtures, and spectroscopy (e.g., Mass Spectrometry or MS, Atomic Absorption Spectroscopy or AAS) for identifying and quantifying substances. These are often used together in powerful hyphenated techniques like GC-MS or LC-MS.
3. How does analytical chemistry impact the pharmaceutical industry? In the pharmaceutical industry, analytical chemistry is essential for every stage of drug development. It's used to identify new drug candidates, ensure the purity and stability of active pharmaceutical ingredients (APIs), and perform dissolution testing to verify a tablet's release properties. These methods are critical for regulatory compliance and ensuring patient safety.
4. Why is data integrity a major concern in analytical labs? Data integrity is a major concern because modern laboratories generate vast amounts of electronic data. Ensuring data is accurate, complete, and protected from unauthorized changes is crucial for compliance with regulations like the FDA's 21 CFR Part 11. It is the foundation of trust in a lab's results, impacting everything from drug approvals to food safety certifications.
















