Scientific discovery relies entirely on the assurance that others can verify findings independently. A crisis in reproducibility challenges the validity and reliability of findings across various domains, particularly in biological research where experimental complexity is high. For laboratory professionals, addressing this issue requires rigorous adherence to technical standards and systematic process controls. Successfully managing these factors ensures that results generated in one laboratory are not only scientifically sound but also consistently attainable by any competent biological laboratory using the same methodology. This commitment to procedural fidelity strengthens the foundation of translational science and accelerates therapeutic development.
Methodological standardization: The cornerstone of reliable results
Defining explicit, step-by-step methods eliminates variation that compromises data quality. Methodological standardization is essential for improving reproducibility in biological research by mitigating subjective interpretation and non-systematic execution. Standard operating procedures (SOPs) must detail every critical step, from sample preparation to final analysis. Laboratory personnel should treat the SOP as the authoritative reference for all procedures, establishing an immutable protocol for the entire workflow.
Key elements for robust SOPs:
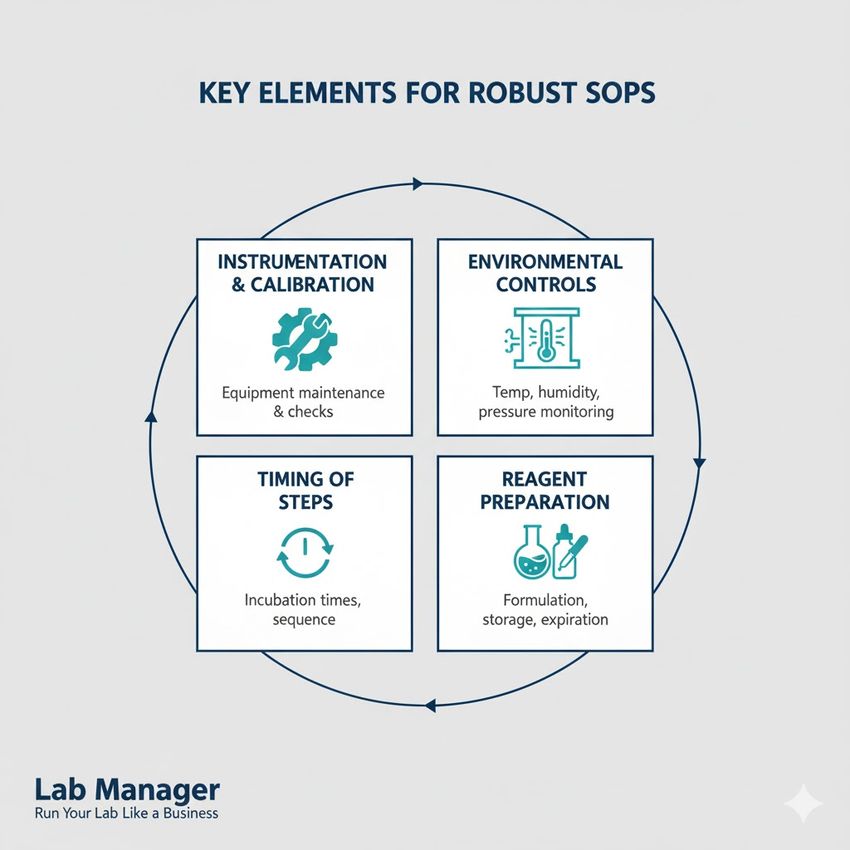
Standard Operating Procedures (SOPs) are only as strong as their foundational details.
GEMINI (2025)
- Instrumentation and calibration: Specify make, model, and serial number of equipment, alongside defined calibration and maintenance schedules.
- Environmental controls: Document temperature, humidity, light exposure, and CO2 levels where samples are handled or incubated.
- Timing of steps: Establish precise incubation periods, mixing times, and centrifugation speeds and durations.
- Reagent preparation: Define molarity, solution formulas, and preparation dates, including specific lot numbers used in validation.
Formal guidance from organizations such as the Clinical and Laboratory Standards Institute (CLSI) provides comprehensive quality management and method validation standards, offering an industry-recognized benchmark for procedural rigor. Adopting such guidelines promotes consistent methodological expectations across laboratories, allowing other researchers to replicate experiments precisely and assess the findings of the biological research.
Reagent validation and sourcing: Mitigating biological variability
Variability in essential reagents, especially biological components like antibodies and cell lines, represents a major threat to reproducibility in biological research. Laboratory personnel must implement stringent qualification and tracking protocols for all critical reagents before use in experimental workflows. A simple substitution of an antibody lot or a change in cell culture media vendor can fundamentally alter an experimental outcome.
Effective reagent management requires attention to three critical areas:
- Sourcing documentation: Document the supplier, lot number, date of receipt, and storage conditions for every batch of critical reagents.
- Functional validation: Before using a new batch or lot, perform functional tests using established positive and negative controls to ensure the reagent performs as expected. For instance, testing a new anti-target antibody lot against known positive and negative cell lines confirms binding specificity and affinity.
- Cell line authentication: Misidentified or contaminated cell lines invalidate an entire body of work. Routine authentication via Short Tandem Repeat (STR) profiling is strongly recommended by the NIH and many journals, and testing for mycoplasma contamination must occur periodically.
Implementing these validation checkpoints drastically reduces the introduction of uncontrolled variables, which in turn elevates the quality of the resulting data and strengthens the potential for reproducibility.
Data management and analysis protocols: Ensuring transparent reporting
Achieving reproducibility extends beyond the bench to the management and interpretation of generated data. A robust data management system ensures data integrity, accessibility, and transparency, which are non-negotiable for high-quality biological research. Poorly organized or inaccessible data archives prevent replication and validation of the original findings.
Key principles for data integrity and management:
- Metadata capture: Log all relevant information (metadata) automatically, including instrument settings, sample identifiers, operator details, and date/time stamps. This contextual information is vital for understanding and recreating the experimental conditions.
- Electronic lab notebooks (ELNs): Mandate the use of ELNs to replace paper records, enabling real-time documentation, secure backup, and detailed version control. ELNs establish a traceable chain of custody for all raw data.
- Adherence to FAIR principles: Data must be Findable, Accessible, Interoperable, and Reusable. Structuring data according to these principles allows automated systems and other researchers to readily locate, understand, and apply the datasets.
- Statistical transparency: Define the statistical methods and exclusion criteria a priori. Report exact p-values, effect sizes, and raw data distributions. Doing this, rather than just using summary statistics, allows others to re-analyze the data and confirm the conclusions drawn from the biological research.
Personnel competency and documentation: Training for rigorous reproducibility
The skills and consistent execution by laboratory personnel significantly influence reproducibility. Even the most detailed SOPs fail if operators lack the necessary training or deviate from the protocol. A comprehensive training program, paired with meticulous documentation, forms a critical pillar of any quality management system in biological research. Training programs must include initial certifications, annual refreshers, and direct observation of performance for high-variability techniques. Documenting training records provides an auditable history of the technical expertise applied to each experiment. Furthermore, a culture of quality encourages proactive identification and correction of potential procedural weaknesses.
Quality assurance and quality control (QA/QC) in biological labs demands constant vigilance and proactive management. QA defines the processes and standards that prevent errors from occurring (e.g., reagent qualification and SOP generation), while QC focuses on the operational techniques used to detect errors after they happen (e.g., running positive/negative controls and evaluating instrument performance checks). The laboratory must establish scheduled reviews of all SOPs and conduct periodic, documented competency assessments for all personnel on complex or high-variability procedures. Only when laboratory professionals consistently execute procedures according to validated standards, and when quality checks yield expected results, does the laboratory truly achieve the level of control necessary for ensuring reproducibility in its biological research. This commitment to documented training and competency verification minimizes the human factor as a source of experimental variation.
Conclusion: Advancing biological research through consistent practice
A structured approach to methodological control, reagent validation, data management, and personnel training drives scientific progress. Enhancing reproducibility in biological research is not merely an aspirational goal but a professional imperative that demands institutional commitment and systematic process implementation from laboratory professionals. Adopting comprehensive quality management principles safeguards the integrity of findings and accelerates the translation of discovery into meaningful scientific advances.
FAQ: Frequently asked questions about achieving reproducibility
What are common barriers to reproducibility in biological research?
Common barriers include undocumented experimental variations, lack of standardization in reagent validation, insufficient personnel training, and incomplete reporting of critical metadata or analytical methods.
How does a robust quality management system support reproducibility?
A robust quality management system supports reproducibility by establishing standardized SOPs (Quality Assurance) and mandatory quality control checks (QC) that ensure consistent performance of methods, instruments, and personnel over time.
What is the role of electronic lab notebooks in improving reproducibility?
Electronic lab notebooks (ELNs) improve reproducibility by providing secure, real-time documentation of all experimental parameters, a traceable chain of custody for data, and version control for protocols, thereby eliminating reliance on subjective paper records.
How often must laboratories authenticate cell lines to ensure reproducibility?
Laboratories must perform routine authentication of cell lines using methods like STR profiling to verify identity and periodically test for contaminants, such as mycoplasma, to ensure the integrity of the biological research.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.














