In the pursuit of justice, the most compelling evidence often exists on a microscopic or molecular scale. A single hair, a minute fiber, a barely visible smudge of paint—these are the silent witnesses that can tell the story of a crime. For laboratory professionals, the challenge lies in giving these witnesses a voice. This is the domain of forensic chemistry, a specialized field of analytical science that provides objective, irrefutable evidence.
Analytical chemistry, at its core, is the science of identifying and quantifying the chemical components of a sample. In a forensic context, this means transforming trace evidence into meaningful data. It is the bridge between a crime scene and a courtroom, ensuring that conclusions are based on rigorous scientific principles rather than conjecture. This article delves into the core analytical techniques that are indispensable to modern forensic science, from separating complex mixtures to identifying the most minute compounds. The methods we will explore are not just theoretical concepts; they are the fundamental tools that empower forensic chemists to uncover the truth and aid in criminal investigations.
Unpacking Trace Evidence: Forensic Chromatography
Chromatography is arguably one of the most vital tools in the forensic chemist’s arsenal. Its fundamental principle is to separate a mixture into its individual components. This is achieved by allowing the sample to move through a stationary phase with the aid of a mobile phase. Different components of the sample interact with the stationary phase in different ways, causing them to move at varying speeds and thus separate from one another. This allows for the identification of each component once it emerges from the system.
Two of the most widely used chromatographic techniques in forensic chemistry are Gas Chromatography-Mass Spectrometry (GC-MS) and High-Performance Liquid Chromatography (HPLC).
Gas Chromatography-Mass Spectrometry (GC-MS): This powerful hybrid technique first separates volatile or semi-volatile compounds using a gas chromatograph (GC). The separated compounds then enter a mass spectrometer (MS), which fragments them and measures the mass-to-charge ratio (m/z) of each fragment. This process generates a unique "mass spectrum" or fingerprint for each compound.
Applications of GC-MS include:
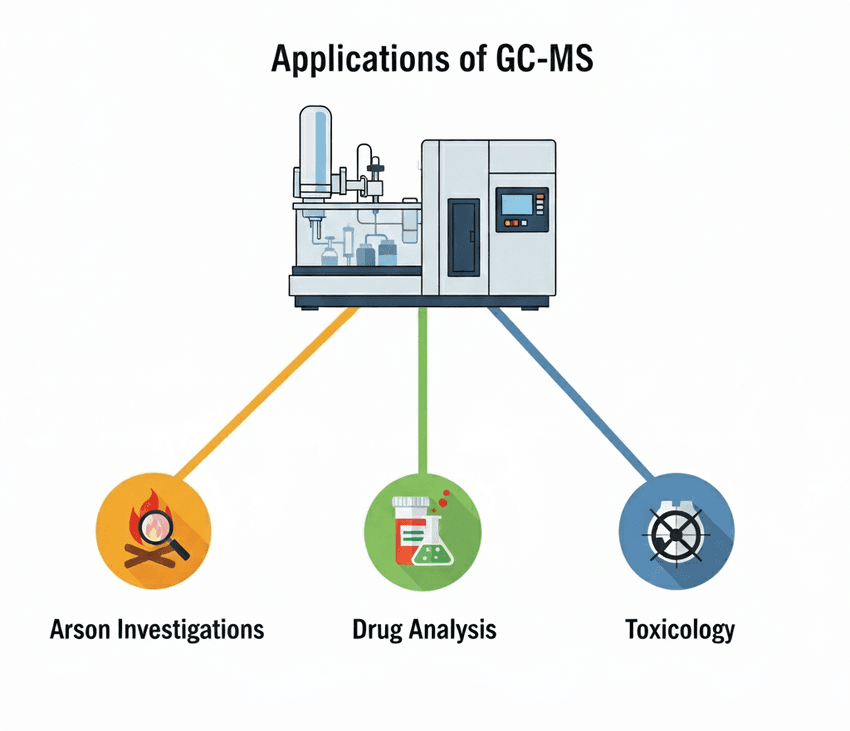
GC-MS has many uses in forensic investigations.
GEMINI (2025)
- Arson Investigations: Analyzing fire debris to identify ignitable liquids like gasoline or kerosene.
- Drug Analysis: Detecting and identifying controlled substances such as heroin, cocaine, and methamphetamine in seized drug samples or biological fluids.
- Toxicology: Quantifying drugs or poisons in blood, urine, or tissue samples.
High-Performance Liquid Chromatography (HPLC): Unlike GC-MS, HPLC is used for non-volatile or thermally unstable compounds. A liquid solvent (the mobile phase) pumps the sample through a column packed with a solid material (the stationary phase). Components separate based on their interaction with the stationary phase.
Applications of HPLC include:
- Forensic Toxicology: Separating and quantifying non-volatile drugs like opioids or antidepressants.
- Explosives Analysis: Identifying trace amounts of explosives like TNT or nitroglycerin.
- Ink and Dye Analysis: Comparing ink from a questioned document to known samples to determine authenticity.
Identifying the Unknown: Forensic Spectroscopic Methods
Spectroscopy involves the study of the interaction between matter and electromagnetic radiation. Different compounds absorb, emit, or scatter light in unique ways, creating a characteristic spectrum that can be used for identification.
Fourier-Transform Infrared (FTIR) Spectroscopy: This technique measures the absorption of infrared light by a sample. Most chemical compounds have a unique IR spectrum that acts like a molecular fingerprint, as specific bonds and functional groups within the molecule vibrate at characteristic frequencies.
Applications of FTIR in forensic labs:
- Fiber Analysis: Identifying the type of polymer in a fiber found at a crime scene.
- Paint Analysis: Comparing the chemical composition of a paint chip from a hit-and-run vehicle to paint from the victim's car.
- Polymer and Plastic Identification: Distinguishing different types of plastics in evidence, such as in drug packaging.
Atomic Absorption (AA) and Emission Spectroscopy: These methods are used to determine the elemental composition of a sample. AA measures the amount of light absorbed by a sample at specific wavelengths, while emission spectroscopy measures the light emitted by a sample when excited.
Applications in forensic chemistry:
- Gunshot Residue (GSR): Identifying characteristic elements like lead (Pb), barium (Ba), and antimony (Sb) on a suspect's hands or clothing.
- Glass and Soil Analysis: Comparing the elemental profile of glass fragments or soil found on a suspect with samples from a crime scene.
The DNA Connection: Electrophoresis in Forensic Chemistry
While chromatography and spectroscopy are powerful for chemical analysis, electrophoresis is the key to one of the most definitive forms of evidence: DNA. Electrophoresis separates charged molecules, such as DNA fragments, based on their size and charge.
Capillary Electrophoresis (CE): Modern forensic DNA analysis relies heavily on CE. It separates DNA fragments amplified via the Polymerase Chain Reaction (PCR) based on size. By comparing the size of specific DNA fragments (known as Short Tandem Repeats, or STRs), forensic scientists can create a unique genetic profile.
Role of CE in modern forensic science:
- Individual Identification: Matching DNA profiles from a crime scene to a suspect or a database like CODIS (Combined DNA Index System).
- Paternity Testing and Victim Identification: Establishing biological relationships or identifying human remains.
- Trace DNA Analysis: Analyzing minute amounts of DNA from objects handled by a suspect.
The precision and discriminatory power of DNA analysis, facilitated by CE, have revolutionized forensic chemistry, providing a level of certainty previously unimaginable.
Definitive Identification: Mass Spectrometry in Forensic Analysis
Mass spectrometry, often used in conjunction with GC or HPLC, stands on its own as a powerful analytical tool. The core principle of a mass spectrometer is to ionize chemical compounds and sort the resulting ions based on their mass-to-charge ratio (m/z). The resulting mass spectrum provides a molecular "fingerprint" that is often definitive for a specific compound.
Beyond its role in hyphenated techniques like GC-MS, mass spectrometry is used in advanced forensic applications. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), for instance, can measure elemental composition down to parts-per-billion levels. This makes it invaluable for analyzing samples where trace elements are key.
Key Applications of Mass Spectrometry:
- Toxicology: Quantitative analysis of drugs or toxins. Unlike simple qualitative detection, MS can determine the exact concentration of a substance in a biological sample.
- Trace Element Analysis: Comparing the elemental composition of things like paint, glass, or soil, providing a definitive link between a suspect and a crime scene.
- Isotope Ratio Mass Spectrometry (IRMS): Analyzing the ratios of stable isotopes within a sample to determine its origin. For example, IRMS can distinguish between different sources of cocaine or pinpoint the geographic origin of a drug sample.
The combination of mass spectrometry with other techniques provides an unparalleled level of specificity and sensitivity, making it a cornerstone of modern forensic chemistry.
Advancing Justice: The Impact of Forensic Chemistry
The integration of advanced analytical chemistry techniques has transformed forensic science from a largely qualitative field to a quantitative and highly reliable one. Chromatography, spectroscopy, electrophoresis, and mass spectrometry are not just laboratory tools; they are instruments of truth.
For laboratory professionals, mastering these techniques is essential for making a meaningful impact. The ability to correctly analyze a minute amount of evidence can be the deciding factor in an investigation. As technology evolves, so too will these methods, pushing the boundaries of what is possible in the pursuit of justice. The future of forensic chemistry promises even greater sensitivity, speed, and automation, further solidifying its role as an indispensable pillar of the legal system.
FAQ: Your Top Questions About Forensic Chemistry
How is analytical chemistry used to solve crimes?
Analytical chemistry is used to identify and quantify the chemical components of evidence. This can include identifying illicit drugs, matching paint chips, analyzing gunshot residue, and comparing DNA profiles to link suspects to a crime scene.
What is the role of a forensic chemist?
A forensic chemist is a specialized analyst who applies chemical principles and techniques to analyze evidence from a crime scene. They operate instruments, interpret data, and provide expert testimony in court, translating complex scientific findings into understandable facts for legal professionals.
Why is mass spectrometry so important in forensic analysis?
Mass spectrometry is crucial because it provides a definitive and highly specific molecular fingerprint for a compound. It confirms the identity of a substance with a high degree of certainty, making it a gold standard for applications in toxicology, drug analysis, and trace evidence.
How is DNA profiling considered a part of forensic chemistry?
DNA profiling is a key part of forensic chemistry because it relies on analytical methods, specifically electrophoresis, to separate and analyze DNA fragments. The process involves chemical reactions (PCR amplification) and instrumental analysis (CE) to generate a genetic profile used for identification.















