The food and beverage industry is in constant pursuit of novel ingredients that are more sustainable, efficient to produce, and functionally superior to their conventional counterparts. This quest has led to the emergence of precision fermentation, a revolutionary technology that harnesses the power of microorganisms to produce specific, high-purity compounds. Unlike traditional fermentation, which uses microbes to create a broader class of products, precision fermentation genetically engineers these microorganisms—such as yeast, bacteria, or fungi—to act as cellular factories, producing a single, targeted ingredient. This process bypasses the need for traditional agriculture or animal farming, offering a scalable and environmentally friendly alternative. For laboratory professionals, precision fermentation represents a frontier of innovation, combining molecular biology, biochemistry, and process engineering. The laboratory is the epicenter of this technology, where the entire production pipeline—from microbial strain development to final product purification and quality assurance—is meticulously controlled. This article provides a comprehensive overview of the scientific principles and laboratory methodologies that underpin precision fermentation and the production of novel ingredients.
Scientific Principles of Precision Fermentation
At its core, precision fermentation is an exercise in applied microbiology and genetic engineering. The process begins with the selection of a host microorganism. Common choices include Pichia pastoris and Saccharomyces cerevisiae (baker's yeast) for their robust growth characteristics and well-understood genetics. These microbes are chosen for their ability to be genetically modified to produce a specific protein, fat, or other complex molecule that would otherwise be difficult or expensive to obtain. The key scientific principle is the introduction of a new gene into the host organism's DNA, which codes for the target ingredient.
This is a stark departure from traditional fermentation, such as that used for bread or beer, where the microbe's natural metabolic pathways are utilized to convert sugars into alcohol and carbon dioxide. In contrast, precision fermentation is a highly controlled and targeted process. The engineered microorganism, housed within a bioreactor, consumes a nutrient-rich media (often a sugar source) and produces the desired ingredient as a result of its engineered metabolic pathway. This technology offers several advantages, including consistent quality, high purity, and the ability to produce ingredients that are identical to those found in nature but without the associated environmental footprint. The lab's role is to ensure that every variable, from the genetic construct to the fermentation conditions, is optimized for maximum yield and efficiency.
Lab Workflows for Strain Engineering
The success of a precision fermentation process hinges on the performance of the microbial strain. The laboratory workflow for strain engineering is a multi-step process that combines cutting-edge genetic tools with meticulous screening and optimization techniques. This work is at the heart of novel ingredient production, transforming a simple microbe into a high-performing bio-factory.
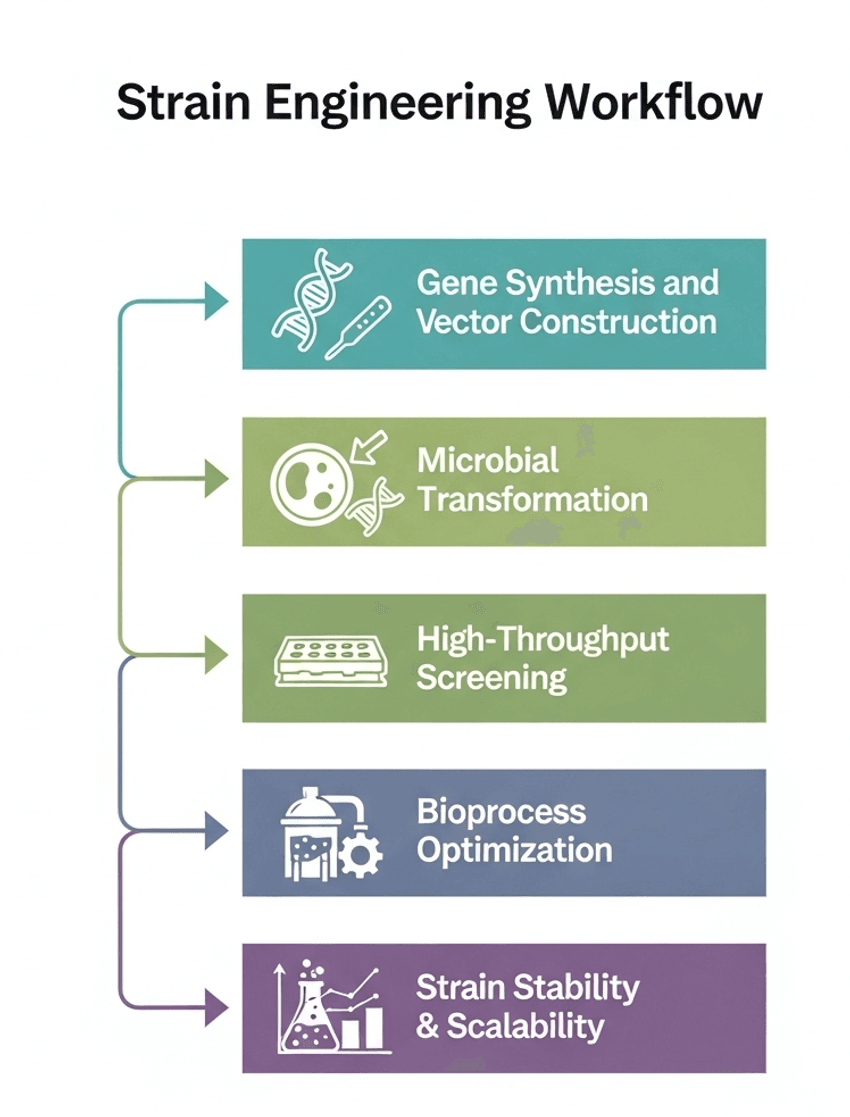
This iterative and data-driven process is what enables precision fermentation to be a reliable and scalable production platform for novel ingredients.
GEMINI (2025)
- Gene Synthesis and Vector Construction: The process begins with the design and synthesis of the gene coding for the target ingredient. This gene is then inserted into a plasmid vector, which serves as the vehicle for introducing the new genetic material into the host microorganism.
- Microbial Transformation: The engineered plasmid is introduced into the host microbe using methods like electroporation or chemical transformation. This process is highly sensitive and requires precise lab protocols to achieve a high rate of successful transformation.
- High-Throughput Screening: After transformation, a large number of microbial colonies are grown and screened to identify the most productive strains. Techniques such as plate assays, fluorescence-activated cell sorting (FACS), or mass spectrometry are used to rapidly quantify the ingredient production of each unique strain.
- Bioprocess Optimization: Once a lead strain is identified, lab-scale bioreactors are used to optimize the fermentation conditions. This involves fine-tuning parameters such as temperature, pH, aeration, and nutrient media composition to maximize the yield of the target ingredient.
- Strain Stability and Scalability: The engineered strain's genetic stability must be verified over multiple generations to ensure consistent performance during large-scale production. Labs also perform scale-up studies to ensure that the fermentation process remains efficient when moving from a lab-bench scale to a commercial bioreactor.
Downstream Processing: From Fermentation to Ingredient
While microbial engineering is crucial, the final product's quality and functionality are determined by the downstream processing. This phase is dedicated to isolating, purifying, and concentrating the target ingredient from the fermentation broth. The lab's role here is to develop and validate protocols that ensure the ingredient meets all purity, safety, and functional specifications.
The downstream process can be complex and is tailored to the specific characteristics of the ingredient. A general workflow often includes:
Step | Purpose | Common Lab Techniques |
|---|---|---|
Cell Harvest | To separate the microbial cells from the fermentation broth. | Centrifugation, filtration |
Cell Disruption | If the ingredient is produced intracellularly, to release the ingredient from inside the cells. | Homogenization, sonication |
Crude Extraction | To separate the target ingredient from other cellular components and media. | Salt precipitation, solvent extraction |
Purification | To achieve high purity by removing impurities and other compounds. | Column chromatography (e.g., ion exchange, affinity), membrane filtration (e.g., ultrafiltration) |
Concentration | To remove water and reduce the volume of the purified ingredient. | Reverse osmosis, evaporation |
Finishing | To produce a stable, final product form (e.g., powder, liquid). | Spray drying, freeze drying |
Every step is monitored and optimized in the lab to minimize product loss and ensure that the final ingredient is safe for consumption and has the desired functional properties. The choice of purification method, for example, is critical for achieving a clean label ingredient free from unwanted residues.
Quality Control and Regulatory Analysis
The final, and arguably most critical, phase of novel ingredient production is rigorous quality control and safety analysis. The laboratory acts as the final gatekeeper, ensuring that the ingredients produced via precision fermentation are safe, consistent, and compliant with all regulatory standards. This is where advanced analytical chemistry techniques come to the forefront.
- Ingredient Purity and Identity: Mass spectrometry, nuclear magnetic resonance (NMR), and HPLC are used to confirm the molecular identity of the ingredient and to quantify any residual impurities.
- Safety Screening: Comprehensive testing is performed to screen for potential contaminants. This includes analyzing for heavy metals using ICP-MS, detecting microbial contaminants via PCR and plate counts, and ensuring the absence of mycotoxins and other toxins.
- Allergenicity and Toxicity: For novel ingredients, labs conduct in-depth analysis to assess potential allergenicity and toxicity. Techniques like ELISA are used to detect potential allergens, while in vitro assays are used to evaluate cytotoxicity.
- Nutritional and Functional Analysis: The final ingredient is analyzed for its nutritional composition, including amino acid profiles, protein content, and vitamin levels. Functional assays are performed to confirm properties like solubility, emulsifying ability, and heat stability, which are critical for food product developers.
This thorough analytical work provides the data necessary for regulatory approval and builds the confidence required for market acceptance of ingredients produced by precision fermentation.
Advancing Food Science with Precision Fermentation
Precision fermentation is a transformative technology that is redefining the landscape of food ingredient production. By leveraging sophisticated laboratory techniques, scientists are able to design and produce a vast array of high-value compounds, from proteins and fats to vitamins and flavors, with unprecedented precision and efficiency. The journey from a genetically engineered microorganism to a market-ready ingredient is a testament to the essential role of the lab in every step of the process—from strain development and fermentation optimization to downstream purification and final product validation. As this technology continues to mature, it will be instrumental in creating more sustainable, resilient, and innovative food systems, ensuring that future generations have access to high-quality nutrition without the environmental cost of traditional production methods.
Frequently Asked Questions About Precision Fermentation
What is the main difference between traditional and precision fermentation?
Traditional fermentation relies on a microbe’s natural metabolic processes to produce a broad class of products (e.g., alcohol, lactic acid). Precision fermentation uses genetically engineered microorganisms to produce a single, specific, and high-purity ingredient.
How are novel ingredients from precision fermentation regulated for safety?
Ingredients from precision fermentation undergo rigorous safety assessments and are subject to regulatory approval by agencies such as the FDA. This involves extensive lab testing to confirm the ingredient's identity, purity, and safety, including tests for contaminants and potential allergens.
What are some examples of ingredients currently produced using precision fermentation?
Examples include whey and casein proteins for dairy-free products, heme protein for plant-based burgers, and fats with specific textural properties, as well as vitamins and flavoring agents.
What is the role of the lab in scaling up precision fermentation?
The lab is critical for scaling up production. It optimizes bioreactor conditions, develops robust downstream processing protocols, and ensures that the quality and consistency of the ingredient are maintained as production moves from a small, lab-scale setting to a large, commercial-scale operation.
















