For decades, the foundation of analytical chemistry and molecular biology has been rooted in the "bulk" measurement of large populations of molecules. Scientists have traditionally worked with ensembles of billions of molecules, collecting data that represents a statistical average. This approach has yielded countless breakthroughs, but it also has a critical limitation: it obscures the unique behavior and heterogeneity that exists at the individual level. In a bulk sample, a small sub-population of highly active or dysfunctional molecules can be completely masked by the average signal of the majority.
Today, a new frontier is emerging that bypasses this limitation. Single-molecule analysis allows laboratory professionals to observe, manipulate, and characterize individual molecules one at a time. This paradigm shift from the average to the individual is providing unprecedented insights into molecular dynamics, reaction pathways, and cellular processes. It is a fundamental change that is already reshaping fields from genomics to clinical diagnostics. This article will explore the core principles, key single-molecule techniques, and transformative applications of this revolutionary field, demonstrating how nanoanalysis is enabling a new era of precision science.
Foundational Principles Driving Single-Molecule Techniques
At its heart, single-molecule analysis is about overcoming the technical challenges of detecting a signal from a single entity amidst a sea of background noise. The principle relies on creating an environment where a single molecule's unique properties—such as its fluorescence, mass, or electrical signature—can be isolated and measured with extreme sensitivity. This level of ultrasensitive analysis requires a combination of specialized instrumentation and meticulous experimental design.
Key principles that underpin these single-molecule techniques include:
- Minimizing Background Noise: The signal from a single molecule is incredibly weak. To detect it, instruments must minimize all sources of background noise, including stray light, electronic noise, and non-specific binding. This often involves working in highly controlled, isolated environments.
- High Sensitivity Detection: Specialized detectors are required to capture the faint signals from individual molecules. Techniques like avalanche photodiodes (APDs) and cooled CCD cameras can capture single photons, making them ideal for fluorescence-based molecular detection.
- Spatial and Temporal Isolation: To ensure that only one molecule is being measured at a time, methods are used to either physically isolate molecules or to observe them in a highly restricted volume. This temporal and spatial isolation is crucial for avoiding signal averaging and obtaining true single-molecule data.
Principle | Traditional Method | Single-Molecule Technique |
|---|---|---|
Molecular Detection | Ensemble averaging of signals from billions of molecules | Direct measurement of an individual molecule's signal |
Sample Volume | Milliliters or Microliters | Femtoliters to Attoliters (picoliter volumes) |
Data Output | Average concentration, kinetics, and conformational states | Distributions of individual behaviors and rare events |
Key Benefit | High-throughput, robust data on populations | Unveiling heterogeneity and dynamic processes |
Implementing Single-Molecule Techniques: Instruments for Nanoanalysis
The shift to single-molecule analysis has been driven by the development of sophisticated instruments and methodologies. These single-molecule techniques allow for direct observation of molecular behavior that was previously hidden.
Fluorescence-Based Methods: This category of single-molecule techniques uses fluorescent labels to make individual molecules "light up" for detection.
- Total Internal Reflection Fluorescence (TIRF) Microscopy: TIRF microscopy illuminates only a very thin layer (less than 100 nm) of the sample near the surface of a coverslip. This dramatically reduces background fluorescence, allowing for clear visualization of single molecules on the surface.
- Fluorescence Correlation Spectroscopy (FCS): FCS measures the fluorescence fluctuations from molecules as they diffuse in and out of a tiny, precisely defined laser focus. By analyzing these fluctuations, researchers can determine diffusion coefficients and concentrations, even at the single-molecule level.
Non-Optical Methods for Nanoanalysis:
- Nanopore Sequencing: This groundbreaking technology uses a tiny pore (nanopore) through which a DNA or RNA strand is passed. As the strand moves through, each base slightly changes the electrical current flowing through the pore. This change in current is unique to each base, enabling direct DNA or RNA sequencing at the single-molecule level without a fluorescent label or amplification.
- Atomic Force Microscopy (AFM): While not exclusively a single-molecule technique, AFM can be used to image the physical shape of individual molecules on a surface. It can also be used to measure the forces involved in molecular interactions, such as protein folding or ligand binding, by pulling on individual molecules.
Applications and Impact of Single-Molecule Analysis on Scientific Research
The ability to perform ultrasensitive analysis at the individual level has far-reaching implications across multiple scientific disciplines. These single-molecule techniques are providing insights that are impossible to gain from bulk measurements.
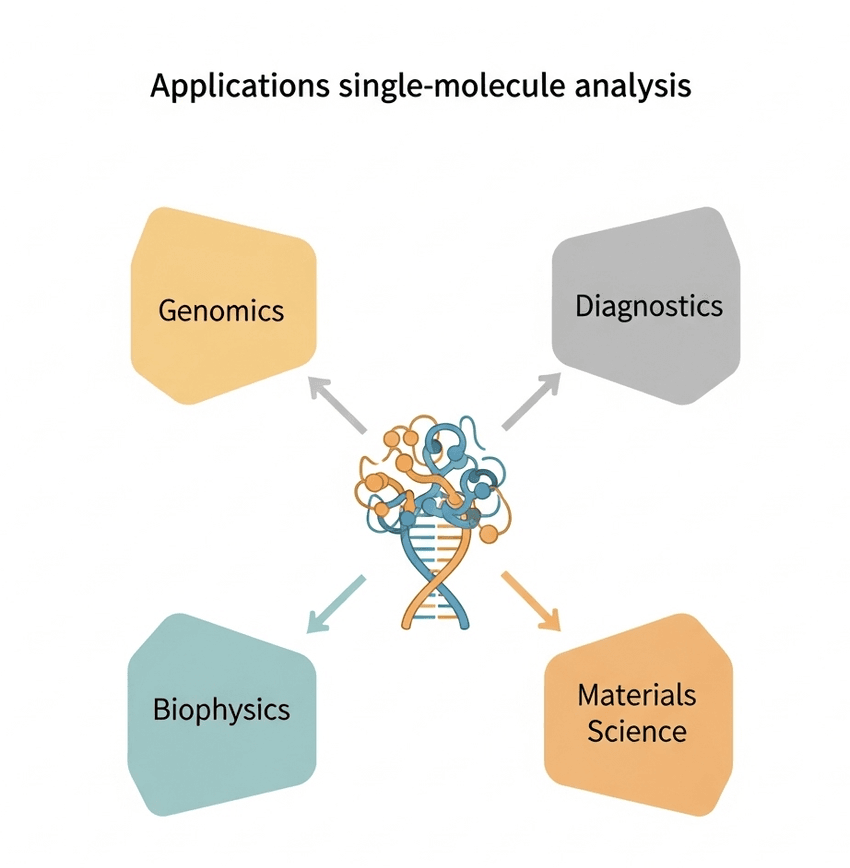
Single-molecule analysis has a variety of uses across many scientific fields.
GEMINI (2025)
- Genomics and Epigenomics: In genomics, single-molecule analysis is a game-changer. Nanopore sequencing enables the sequencing of very long DNA strands, which is critical for mapping complex genomes and detecting structural variations. It also allows for direct detection of epigenetic modifications, such as DNA methylation, without chemical conversion steps.
- Clinical Diagnostics: The extreme sensitivity of these methods holds immense promise for early disease detection. For example, single-molecule detection of rare biomarkers in blood could lead to non-invasive cancer diagnostics long before the disease is detectable by traditional methods. This has the potential to transform personalized medicine.
- Biophysics and Enzyme Kinetics: Single-molecule techniques allow biophysicists to observe the conformational changes of single proteins in real time. For enzyme kinetics, researchers can watch a single enzyme molecule as it processes its substrate, revealing different kinetic states and uncovering heterogeneity within a population of what were once thought to be identical enzymes. This level of detail is critical for understanding biological processes and for drug discovery.
- Materials Science: In materials science, nanoanalysis is used to study the properties of individual polymer chains, nanoparticles, or quantum dots. Understanding the behavior of these building blocks on an individual level can lead to the design of new materials with superior and more predictable properties.
The Future of Ultrasensitive Analysis is Here
The field of single-molecule analysis is rapidly evolving, with new techniques and applications emerging constantly. While challenges remain—such as improving throughput and developing robust data analysis pipelines—the progress is undeniable. The ability to directly observe the unique behavior of a single molecule is fundamentally changing how we approach scientific inquiry.
The future of science is one where ultrasensitive analysis becomes a routine practice in many labs. As instruments become more automated and affordable, and data analysis software becomes more user-friendly, the power of single-molecule techniques will move beyond specialized research labs and into clinical and industrial settings. This will lead to a new era of personalized medicine, more efficient drug development, and a deeper understanding of the molecular world.
Frequently Asked Questions about Single-Molecule Analysis
What is the main difference between traditional and single-molecule analysis?
Traditional analysis measures the average behavior of a large population of molecules, while single-molecule analysis measures the unique properties of individual molecules. This allows researchers to uncover heterogeneity and observe rare events that would be hidden in bulk measurements.
What are some key single-molecule techniques used today?
Common single-molecule techniques include fluorescence-based methods like TIRF microscopy and FCS, as well as non-optical methods such as nanopore sequencing and atomic force microscopy, which are all part of the growing field of nanoanalysis.
How does single-molecule analysis improve clinical diagnostics?
The extreme sensitivity of single-molecule detection allows for the identification of rare biomarkers in samples like blood or urine. This capability is crucial for early disease detection, particularly in conditions like cancer, where a small number of disease-related molecules may be present long before symptoms appear.
What is a major challenge in implementing single-molecule techniques?
One of the primary challenges is dealing with the low signal-to-noise ratio inherent in measuring a single entity. It requires specialized, expensive equipment and careful experimental design to ensure reliable and accurate ultrasensitive analysis.