Bioluminescence detection is a cornerstone of modern cell-based assays, enabling researchers to monitor and quantify a broad range of biological processes in real time. Recently, researchers have begun using bioluminescence imaging to illuminate the spatiotemporal context in which these processes take place. This data is critical for understanding localization patterns, cell-to-cell variability, and much more.
For researchers working with bioluminescent assays, an affordable and easy-to-use bioluminescence imaging system could provide additional insights beyond what can be captured by bulk signal measurements. Choosing the right instrument depends on several key considerations to ensure success.
Beyond plate-based assays
Bioluminescent plate-based assays have become widely popular in a broad range of functional biology applications, including gene expression profiling, pathway analyses, protein-protein interaction studies, and high-throughput screening.1 Unlike fluorescence, which requires high-intensity external light for excitation, bioluminescence is a self-emitting enzymatic reaction. A luciferase enzyme catalyzes the oxidation of a luciferin substrate, resulting in a light signal. Given their inherently low background and non-toxic nature, bioluminescent assays offer exceptionally high signal-to-noise ratios, broad dynamic ranges, and compatibility with long-term kinetic measurements.1, 2
Conventional plate-based assays typically report bulk luminescent readouts from multi-well plates, measuring average signal intensities across entire cell populations. While these assays are valuable in many applications, they also have several shortcomings. Plate-based assays lack important spatial context, such as whether a reporter is uniformly expressed in a cell population, whether a target protein is properly localized, or whether subpopulations of cells respond differently to the same stimulus. They also offer no insight into how signals vary from cell to cell or within individual cells.
Bioluminescence imaging (BLI) addresses these limitations by enabling direct visualization of luminescent signals at the single-cell level. This opens the door to more detailed biological questions: Is the reporter expressed uniformly in all the cells? Is the reporter restricted to a specific organelle? Does signal localization shift after stimulation? Do all cells behave the same way? BLI extends the capabilities of traditional bioluminescent assays beyond population-average measurements by providing single-cell spatially resolved insights.
Advantages of bioluminescence imaging
Most cellular imaging has historically depended on fluorescence. Fluorescence imaging is often favored for its high photon output and rapid acquisitions, which enable short exposure times and high-resolution imaging. While this is useful for fixed-cell imaging or rapid dynamics processes, fluorescence imaging has well-known limitations in live-cell contexts.3
Autofluorescence from cellular components or culture media can obscure weak signals, and photobleaching can gradually diminish signal intensity over time. Most importantly, repeated excitation can generate reactive oxygen species, leading to phototoxicity that compromises cell viability or perturbs biological processes. These challenges are particularly pronounced in live-cell experiments requiring prolonged kinetic measurements.3
Bioluminescence offers a compelling, low-background, excitation-free alternative capable of detecting proteins across a broad expression range, including at endogenous levels. Although bioluminescent systems require longer exposures due to significantly lower photon output, the lack of excitation light reduces biological perturbation, and helps to preserve cell viability during prolonged experiments.2, 3 This makes BLI especially well-suited for real-time imaging of dynamic processes such as gene expression, protein degradation, signal transduction, and protein trafficking under physiologically relevant conditions.
Despite these advantages, BLI remains limited in spatiotemporal resolution. Longer acquisition times can hinder capture of rapid or transient events and may reduce resolution of fine subcellular features.3 For applications that demand rapid acquisition or high-resolution localization, such as super-resolution microscopy or sub-organelle tracking, fluorescence remains the preferred modality.
Choosing the right bioluminescent imaging system
For researchers already using bioluminescent assays in plate-based formats, adopting a compatible imaging system can expand their experimental capabilities. Many common bioluminescent assays can be imaged with minimal modifications to existing protocols. The key is choosing an imaging system equipped with features that support the intended biological applications. Technical features to consider include:
- Environmental control: Live-cell imaging often requires maintaining cells in stable, physiologically relevant conditions for extended periods of time. Imaging systems with a built-in environmental chamber that control for temperature and CO₂ levels allow researchers to perform continuous time-lapse imaging over hours or even days without compromising cell health.
- Filtered luminescence detection: Assays involving bioluminescence resonance energy transfer (BRET), including those probing protein-protein interactions and target engagement, depend on filtered luminescence detection to resolve donor and acceptor emissions. These are typically separated using integrated bandpass or longpass filters within the imaging system.
- Multi-modal imaging: The ability to overlay bioluminescence with brightfield or fluorescence images can enhance spatial interpretation. Brightfield imaging helps locate cells and establish morphology, while fluorescence can highlight subcellular structures. For instance, co-localization of a bioluminescent signal with fluorescent-based organelle markers (e.g., for the nucleus or mitochondria) can reveal whether a protein is properly expressed or redistributed during signaling events. In addition, brightfield and fluorescence channels can also aid in focusing, especially when working with dim bioluminescent signals.
In addition to technical capabilities, usability plays a crucial role in determining how easily researchers can incorporate bioluminescence imaging into their workflows. Systems that are intuitive and easy to operate can reduce the learning curve and help researchers start imaging quickly, even without prior experience in advanced microscopy. Features that support ease of use include:
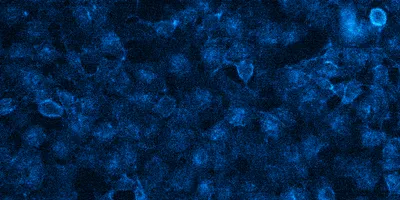
Bioluminescence image of Genome edited HEK293 cells expressing NanoLuc-BRD4, which is localized to the nucleus. Upon treatment with substrate, image was taken using the GloMaxTM Galaxy bioluminescent imager with a 90-second exposure time.
Credit: Promega
- Simplified software interface: A user-friendly interface makes it easy for researchers at all experience levels to acquire high-quality images. One of the most common practical challenges is focusing, particularly when working with low-expression targets. While bright signals are easy to focus on, dim signals may require additional care. Many users quickly develop effective focusing strategies such as relying on brightfield or fluorescence channels.
- All-in-one design: Integrated systems eliminate the need to modify fluorescence microscopes with custom bioluminescent modules. This reduces cost, complexity, and time to the first experiment.
- Out-of-the-box readiness: Systems that do not need professional installation often allow users to begin experiments with minimal setup.
A closer look at biological processes
Bioluminescence imaging offers a practical and useful extension to traditional plate-based bioluminescent assays. It enables researchers to visualize biological processes with spatial and temporal resolution, while maintaining the core advantages of bioluminescence detection, including low background, high sensitivity, and kinetic compatibility. With the right imaging system equipped for environmental control, filtered detection, and multi-modal imaging, researchers can gain deeper insight into dynamic cellular processes without significant changes to existing workflows. As imaging platforms continue to become more user-friendly and versatile, bioluminescence imaging is poised to play an increasing role in the study of dynamic biological processes.
References:
1. Roda, B.; Deo, S. K.; O’Connor, G.; Moraskie, M.; Giordani, S.; Marassi, V.; Roda, A.; Daunert, S. Shining light on biosensors: Chemiluminescence and bioluminescence inenabling technologies. TrAC Trends in Analytical Chemistry 2024, 180.
2. Love, A. C.; Prescher, J. A. Seeing (and Using) the Light: Recent Developments in Bioluminescence Technology. Cell Chem Biol 2020, 27 (8), 904–920.
3. Bauer, C. R. Bioluminescence Microscopy New Avenues in Live Cell Imaging. G.I.T. Imaging & Microscopy 2013, 4, 32–34.