Material verification remains a cornerstone of analytical excellence in modern laboratory environments. XRF/XRD validation for purity and composition serves as a critical checkpoint. It ensures that raw materials and finished products meet stringent quality standards. Laboratory professionals rely on these complementary non-destructive techniques to identify elemental constituents and crystallographic structures with high precision. Regulatory bodies increasingly mandate robust validation data to support safety claims and efficacy. This requirement is particularly strict in pharmaceutical and advanced materials sectors. Mastering the integration of X-ray fluorescence (XRF) and X-ray diffraction (XRD) into routine quality control workflows minimizes the risk of batch failures. It also ensures compliance with global standards.
Principles of XRF and XRD for purity and composition analysis
Understanding the distinct mechanisms of X-ray interaction with matter allows laboratories to leverage the full potential of these analytical tools. XRF and XRD operate on fundamental physical principles that yield different but complementary datasets regarding a sample's identity.
X-ray fluorescence (XRF) analysis determines elemental composition by measuring the fluorescent X-rays emitted from a sample when excited by a primary X-ray source. Each element produces a unique set of characteristic fluorescent X-rays, which effectively creates a fingerprint for that specific element. The intensity of these emissions correlates directly to the concentration of the element within the sample. This technique excels in detecting elemental impurities and major constituents across a wide dynamic range, from parts per million (ppm) to high percentage levels. Energy Dispersive X-ray Fluorescence (EDXRF) and Wavelength Dispersive X-ray Fluorescence (WDXRF) represent the two primary configurations, with WDXRF offering superior resolution for complex matrices.
X-ray diffraction (XRD), conversely, investigates the crystalline structure of materials. When a monochromatic X-ray beam strikes a crystalline sample, the lattice planes diffract the beam at specific angles defined by Bragg’s Law. The resulting diffraction pattern acts as a definitive identifier for crystalline phases, making XRD indispensable for distinguishing between polymorphs, salts, and solvates of the same chemical compound. While XRF answers the question "which elements are present," XRD answers "how are these elements arranged."
Key differences between XRF and XRD:
- Target analysis: XRF analyzes elemental composition; XRD analyzes crystal structure and phase composition.
- Detection capability: XRF typically detects elements from Sodium to Uranium (with lighter elements like Beryllium possible using specialized optics); XRD identifies crystalline compounds and phases.
- Sample state: Both techniques handle solids, powders, and thin films, though XRF can also analyze liquids.
- Primary application: XRF suits elemental quantification; XRD suits phase identification and polymorphism analysis.
Successful XRF/XRD validation for purity and composition depends on recognizing these distinctions. A comprehensive validation strategy often employs both methods to confirm that a material not only contains the correct chemical elements in the right proportions but also exists in the desired solid-state form.
Implementing XRF/XRD validation protocols for quality control
Robust validation protocols establish the reliability and reproducibility of analytical data generated by X-ray instrumentation. Establishing a validation framework requires a systematic approach that aligns with guidelines from organizations such as the International Conference on Harmonisation (ICH) and the United States Pharmacopeia (USP).
The process begins with Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These stages verify that the instrument is installed correctly, operates within specified limits, and performs consistently under routine working conditions. For XRF/XRD validation for purity and composition, the PQ phase is particularly critical. It involves running reference materials with known properties to demonstrate that the system produces accurate and precise results over time. Laboratories must define acceptance criteria for parameters such as linearity, accuracy, precision, specificity, and limits of detection.
Reliable X-ray Fluorescence (XRF) and X-ray Diffraction (XRD) results depend on rigorous method validation
GEMINI (2026)
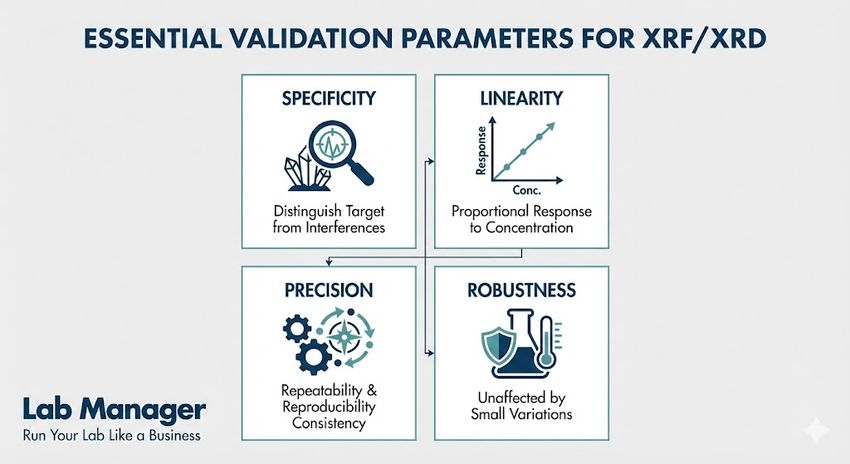
Essential validation parameters:
- Specificity: The ability to assess the analyte unequivocally in the presence of components like impurities or excipients. In XRD, this involves distinguishing a specific polymorph from others.
- Linearity: The ability to obtain results directly proportional to the concentration of the analyte. XRF calibration curves must demonstrate high correlation coefficients.
- Precision: The measure of agreement among individual test results. Repeatability and intermediate precision studies confirm instrument stability.
- Robustness: The capacity of the method to remain unaffected by small, deliberate variations in method parameters, such as slight changes in sample position or X-ray tube voltage.
Data integrity plays a critical role at this stage. Modern software packages for XRF and XRD often include features to support compliance with 21 CFR Part 11, ensuring that electronic records remain secure and traceable. Audit trails, user access controls, and data versioning prevent unauthorized alterations to validation results. Laboratories must document every step of the validation lifecycle, creating a comprehensive evidence trail that withstands regulatory scrutiny.
Applications of XRF/XRD validation in pharmaceutical and industrial analysis
Diverse industries utilize X-ray analysis to solve complex quality challenges and ensure product safety. The versatility of XRF and XRD allows them to address specific regulatory requirements across sectors ranging from pharmaceuticals to cement manufacturing.
In the pharmaceutical industry, the control of solid-state forms constitutes a primary application of XRD. Active pharmaceutical ingredients (APIs) often exist in multiple crystalline forms, or polymorphs, which exhibit different solubility, stability, and bioavailability profiles. XRD serves as the gold standard for polymorph screening and monitoring. Validation methods here focus on the detection limit of minority phases to ensure that an undesirable polymorph does not contaminate the final product. XRF complements this by monitoring elemental impurities, such as catalysts (palladium, platinum) or environmental contaminants (lead, arsenic), in accordance with ICH Q3D guidelines. XRF/XRD validation for purity and composition thus acts as a dual shield, safeguarding both the chemical purity and the physical efficacy of the drug.
Industrial applications, such as metallurgy, mining, and cement production, prioritize high-throughput process control. In cement manufacturing, for instance, the phase composition of clinker directly dictates the setting time and strength of the final concrete. On-line XRD analyzers provide real-time feedback on mineral phases like alite and belite, allowing operators to adjust kiln parameters instantly. Similarly, XRF validates the elemental composition of alloys in metal foundries, ensuring that steel grades meet strict ASTM specifications.
Industry-specific validation focus areas:
Industry | Primary XRF Application | Primary XRD Application | Key Regulatory Reference |
|---|---|---|---|
Pharmaceuticals | Elemental impurity analysis (Class 1, 2A, 2B elements) | Polymorph identification and quantification | |
Mining & Minerals | Ore grading and trace element analysis | Mineralogical phase quantification | |
Cement | Oxide analysis (CaO, SiO2, Al2O3) | Clinker phase monitoring (Alite, Belite) | |
Environmental | Heavy metal screening in soil and waste | Identification of hazardous asbestos minerals |
These examples illustrate that while the core physics remains constant, the validation strategy adapts to the specific risks and requirements of the application. A method validated for mining ore grading might lack the sensitivity required for pharmaceutical impurity testing, highlighting the need for fit-for-purpose validation protocols.
Role of certified reference materials in XRF/XRD validation
Certified Reference Materials (CRMs) act as the anchor point for all analytical measurements in X-ray spectrometry validation. Without these traceable standards, laboratories cannot guarantee the accuracy of their XRF/XRD validation for purity and composition. CRMs provide a known value with an associated uncertainty, allowing analysts to calibrate instruments and verify method bias. In XRF analysis, matrix-matched CRMs are essential because the sample matrix absorbs and enhances X-rays, influencing the signal intensity. Using a CRM with a matrix similar to the unknown sample corrects for these inter-element effects. For XRD, standards such as silicon powder or corundum assist in aligning the goniometer and determining instrumental broadening. Regular analysis of CRMs as part of a quality control chart detects drift in instrument performance before it impacts data quality. This practice aligns with ISO/IEC 17025 requirements for metrological traceability, ensuring that laboratory results are comparable internationally.
Conclusion on XRF/XRD validation for purity and composition
XRF/XRD validation for purity and composition remains an indispensable component of modern laboratory quality assurance. By understanding the distinct physical principles of X-ray fluorescence and diffraction, laboratory professionals can design robust validation protocols that address both elemental and crystallographic requirements. The implementation of rigorous IQ/OQ/PQ procedures, combined with the use of certified reference materials and compliant software, ensures data integrity and regulatory adherence. As industries continue to demand higher purity and tighter process control, the reliance on these non-destructive techniques will only grow. Laboratories that prioritize comprehensive validation strategies position themselves to deliver accurate, reliable results that safeguard product quality and consumer safety.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.












