Laboratories processing forensic evidence or clinical specimens frequently encounter biological materials with unknown pathogenic potential. Safe management relies heavily on establishing robust biohazard and toxin containment strategies that protect personnel without compromising evidence integrity. Proper sample handling protocols serve as the primary defense against laboratory-acquired infections (LAIs) and accidental environmental release. This article outlines the essential procedures, engineering controls, and risk assessment frameworks necessary for maintaining high safety standards in analytical and forensic settings.
Principles of risk assessment and biological safety levels
Risk assessment dictates the operational parameters for handling dangerous biological agents and toxins.
Every laboratory must perform a comprehensive risk assessment before initial work with a new agent or sample type begins. This process involves identifying hazards in known agents or assessing potential risks in unknown forensic samples. The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) categorize agents into four Risk Groups (RG) based on pathogenicity, mode of transmission, host range, and treatment availability. Risk Groups classify organisms; Biosafety Levels define facility requirements, and although related, they are not interchangeable.
Proper classification guides the selection of the appropriate Biosafety Level (BSL). Most routine clinical and forensic work involving sample handling of blood, body fluids, or tissue occurs at BSL-2, which requires specific containment devices and administrative controls. However, clinical labs may encounter pathogens requiring escalation to BSL-3 (e.g., Mycobacterium tuberculosis). Facilities reserve BSL-4 for dangerous and exotic agents posing a high individual risk of aerosol-transmitted laboratory infections and life-threatening disease.
For biological toxins, the risk assessment must account for the specific lethal dose (LD50), the physical state of the toxin (solution vs. powder), and manipulation procedures. Dry powders pose significant inhalation risks and static electricity issues, requiring specialized handling techniques often exceeding standard BSL-2 precautions. The "Biosafety in Microbiological and Biomedical Laboratories" (BMBL) serves as the definitive reference for determining these containment levels in the United States.
Engineering controls and personal protective equipment
Primary barriers protect the operator and the immediate environment from exposure to biohazards and toxins.
Engineering controls isolate the hazard from the worker. The Class II Biological Safety Cabinet (BSC) remains the cornerstone of containment in BSL-2 and BSL-3 laboratories. These cabinets utilize High-Efficiency Particulate Air (HEPA) filters to create a sterile work environment while preventing aerosol escape. Proper sample handling within a BSC involves specific techniques: separating clean and dirty zones, minimizing rapid movements that disrupt the air curtain, and utilizing absorbent pads to contain minor spills.
When handling biological toxins, particularly those with low LD50 values like botulinum neurotoxins or ricin, laboratories often employ glove boxes or Class III BSCs to provide a physical barrier between the operator and the material. This prevents potential aerosolization during centrifugation, grinding, or blending.
Personal Protective Equipment (PPE) acts as the last line of defense. The selection of PPE depends on the risk assessment. Standard precautions for handling biohazard materials include fluid-resistant laboratory coats, eye protection (safety glasses or face shields), and disposable gloves. Nitrile gloves generally offer better chemical resistance and reduced allergy risk compared to latex. For high-risk procedures or toxin handling, double-gloving allows operators to remove the outer layer immediately upon contamination while maintaining skin protection. Operators require respiratory protection, such as N95 respirators or Powered Air-Purifying Respirators (PAPRs), when engineering controls cannot capture all potential aerosols or when working with specific respiratory pathogens.
Protocols for secure sample transport and storage
Maintaining containment during material movement prevents accidental exposure and environmental contamination.
Transporting hazardous materials within the facility requires durable, leak-proof secondary containers. These containers must securely hold the primary sample tubes and contain sufficient absorbent material to capture the entire liquid volume in case of breakage. Staff must disinfect the secondary container surface before it leaves the laboratory area to prevent contaminant spread to clean zones like hallways or administrative offices.
External transport dictates strict adherence to Department of Transportation (DOT) and International Air Transport Association (IATA) regulations. Biological substances generally fall into Category A (infectious substances affecting humans/animals, UN 2814 or UN 2900) or Category B (biological substances, UN 3373). Category A shipments require rigid triple packaging that has passed stringent performance testing, including drop and pressure tests.
Triple packaging system components:
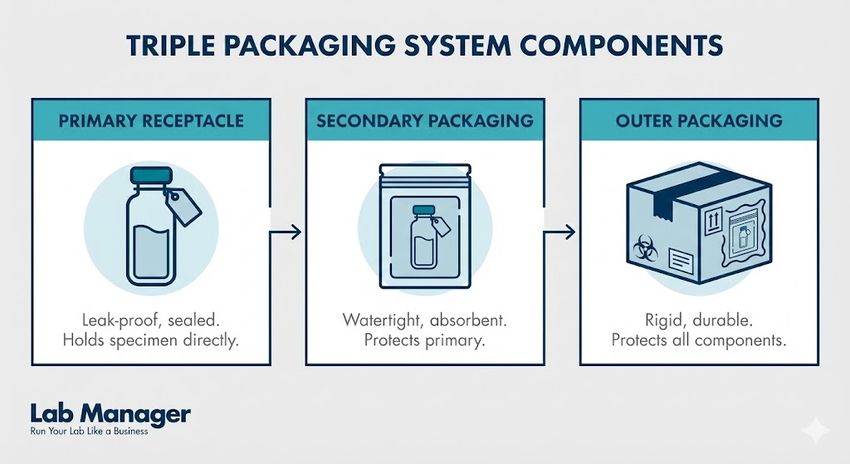
Adhering to the "Triple Packaging System" is critical for maintaining sample integrity and ensuring the safety of personnel during transport.
GEMINI (2025)
- Primary receptacle: A leak-proof vessel containing the specimen.
- Secondary packaging: A leak-proof, impact-resistant container with absorbent material.
- Outer packaging: A rigid container with proper hazard labeling and shipping documentation.
Chain of custody acts as a critical component of sample handling in forensic laboratories. Documentation must track every individual who handles the biohazard or toxin evidence. This tracking ensures legal admissibility and confirms that storage conditions (such as temperature logs) prevented degradation. Security measures, including restricted access freezers and lockable storage boxes, prevent unauthorized access or tampering with sensitive biological evidence.
Decontamination procedures and waste management strategies
Effective decontamination neutralizes biological threats before disposal or equipment reuse.
Decontamination encompasses sterilization, disinfection, and antisepsis. The method choice depends on the target organism, organic load, and material requiring treatment. Autoclaving (steam sterilization) serves as the most reliable method for inactivating biological waste. Validation of autoclave cycles using biological indicators (such as Geobacillus stearothermophilus spores) ensures the equipment achieves the necessary temperature and pressure to kill resistant agents.
Chemical disinfection plays a vital role in surface decontamination and liquid waste treatment. Sodium hypochlorite (household bleach) acts as a broad-spectrum disinfectant effective against bacteria, viruses, and many toxins. However, bleach degrades DNA, posing a unique challenge for forensic laboratories attempting to preserve genetic profiles. In these contexts, personnel must carefully manage alternative disinfectants or specific contact times.
Spill response protocol overview:
- Alert personnel: Notify nearby staff and restrict access to the area.
- PPE application: Don appropriate protective gear before approaching the spill.
- Containment: Cover the spill with absorbent towels to prevent spreading.
- Disinfectant application: Pour an appropriate disinfectant over the towels, starting from the perimeter and working inward.
- Contact time: Allow sufficient time (typically 15–30 minutes) for the disinfectant to act.
- Disposal: Discard materials as hazardous waste and clean the area with fresh disinfectant.
Toxin inactivation often requires more aggressive chemistry. For example, safety protocols often recommend sodium hypochlorite combined with sodium hydroxide for inactivating protein toxins like ricin. Laboratories must maintain Safety Data Sheets (SDS) and specific inactivation protocols for every toxin present in the inventory.
Forensic laboratory considerations for hazardous evidence
Forensic laboratories face unique challenges where safety imperatives directly compete with the need to preserve sample integrity. Unlike clinical settings where a sample might be replaceable, forensic evidence is often finite and unique. Unknown powders, dried blood stains on weapons, or tissues from decomposing remains present undefined biohazard risks. Personnel often must process these items to recover trace DNA or chemical signatures. This necessitates a workflow where sample handling minimizes cross-contamination (which ruins the DNA profile) and protects the analyst from potential pathogens like Hepatitis B, HIV, or anthrax. Protocols mandate DNA-free cleaning agents to prevent introducing exogenous DNA, but these agents must also effectively neutralize biological risks. Furthermore, chemical cutting agents in drug samples (like fentanyl) create a dual-hazard environment requiring chemical fume hoods or powder-containment hoods alongside biological safety measures.
Conclusion regarding hazardous material management
Effective management of biohazard and toxin materials demands a disciplined approach to laboratory safety. By strictly adhering to risk assessment protocols, utilizing appropriate engineering controls, and maintaining rigorous sample handling procedures, laboratories protect their most valuable asset: their personnel. Continuous training and strict compliance with regulatory standards ensure that scientific inquiry and forensic analysis proceed without compromising human health or environmental safety.
FAQ
What distinguishes a biohazard from a chemical hazard?
A biohazard refers to biological substances that pose a threat to the health of living organisms, primarily humans, such as viruses, bacteria, and medical waste. Chemical hazards cause harm through toxicity, corrosiveness, or flammability rather than infection.
How does the chain of custody impact sample handling protocols?
Chain of custody requires documentation of every transfer and storage location of a sample to prevent tampering and ensure legal admissibility. This adds a layer of administrative complexity to safety protocols, as all handlers must be documented and authorized.
What is the correct procedure for handling unknown powdery substances in a forensic lab?
Unknown powders require handling within a certified containment device, such as a Class II BSC or a chemical fume hood, depending on the suspected risk. Field testing should occur only with appropriate PPE to rule out immediate threats like anthrax or fentanyl before confirmatory testing.
Why is risk assessment critical before starting new experiments?
Risk assessment identifies specific hazards associated with an agent or procedure and determines the necessary biosafety level and PPE. This proactive step prevents exposure incidents by aligning safety measures with the actual risk level of the work.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.














