Laboratory professionals oversee complex, high-stakes operations that demand rigorous financial planning. The effective management of laboratory budgets and the successful acquisition of extramural grants directly determine research continuity and operational stability. Sound financial stewardship ensures resources align with scientific objectives, from procuring specialized reagents to maintaining advanced instrumentation. Mastering these financial disciplines transforms a facility's operational capabilities. It allows scientists to focus on high-impact research outcomes rather than resource constraints. This proactive approach to financial health is indispensable for any modern biological research environment.
Establishing rigorous budgeting principles and effective financial forecasting
Effective financial planning relies on granular data and forward-looking analysis. Establishing rigorous budgets involves categorizing and forecasting expenditures across fixed and variable operational costs.
Fixed costs typically include personnel salaries, long-term instrument leases, service contracts, and facility-related expenses such as rent or utility base rates. These costs show minimal fluctuation regardless of the facility's immediate activity level. Financial managers must model personnel costs accurately. This modeling incorporates projections for wage inflation, benefits overhead, and planned staff increases or reductions.
Variable costs encompass resources directly tied to experimental throughput, such as specialized consumables, reagents, tissue culture media, animal models, and maintenance schedules triggered by usage rather than time. Accurate financial forecasting requires modeling potential fluctuations in experimental needs and supply chain volatility. This step is crucial for preventing unexpected shortfalls in the budgets. Managers should implement rolling forecasts. Expenditure projections update monthly based on real-time consumption data from inventory management systems. Furthermore, integrating safety and compliance costs into fiscal planning is advised by the guidance provided by the Occupational Safety and Health Administration (OSHA).
A key strategic principle involves zero-based budgeting (ZBB), applied periodically to major sections of the budget. ZBB requires managers to justify every expenditure anew, starting from a metaphorical zero base, rather than simply adjusting previous figures. This critical process ensures alignment between financial allocation and the most current scientific priorities of the biological facility. Financial managers must also align practices with broader public health budgeting guidelines, such as those detailed by the World Health Organization (WHO) in its Laboratory Quality Management System documentation. Additionally, the application of these techniques reflects the need for sustainable resource management and cost justification, a widely recognized best practice. Separating capital expenditure (CapEx) budgets from operational expenditure (OpEx) budgets ensures proper accounting. CapEx funds the acquisition of major assets like new flow cytometers or imaging systems. OpEx provides adequate funding for daily scientific work.
Strategic grant acquisition and the grant lifecycle management
Securing external grants provides the vital capital required for ambitious research projects. However, managing them demands a specialized approach across the entire grant lifecycle. This management approach should align with the professional standards set by organizations like the Association of Research Managers and Administrators (ARMA).
The lifecycle begins with strategic planning, which identifies funding opportunities that align precisely with the research mission of the biological facility. Proposal development involves meticulous budget justification. This step clearly demonstrates that all requested funds are necessary, reasonable, and allocable solely to the stated project aims. Pre-award grant administration focuses heavily on adherence to the funding agency’s caps. Managers must ensure the proposed budget follows guidelines for indirect costs and salary rates. This prevents potential delays or mandatory reductions later in the process.
Once awarded, post-award management shifts focus to strict adherence to the sponsor's financial regulations. For federal awards, this requires rigorous compliance with the U.S. Department of Health and Human Services (HHS) Uniform Administrative Requirements (2 CFR 200). This adherence includes detailed effort reporting, mandatory for personnel paid from federal grants, which verifies that staff time matches the funding source. Other critical areas include tracking cost transfers, managing equipment inventory purchased with grant funds, and recovering indirect costs according to the negotiated federal rate. The National Institutes of Health (NIH) Grants Policy Statement emphasizes the need for responsible stewardship of these federal research funds. Maintaining comprehensive documentation of all expenditures throughout the grant period minimizes the risk of audit findings. This practice also ensures compliance with institutional and federal guidelines. Effective management of awarded grants ensures the facility maximizes the scientific output delivered per funding dollar.
Lab Management Certificate
The Lab Management certificate is more than training—it’s a professional advantage.
Gain critical skills and IACET-approved CEUs that make a measurable difference.
Navigating compliance, audit preparedness, and regulatory overhead
Biological facilities operate under numerous local, national, and international regulatory frameworks. These frameworks impose significant financial and administrative overhead. Budgeting must explicitly account for costs associated with maintaining compliance.
These compliance-driven expenses are often overlooked during initial planning but prove essential for continued operation. They include:
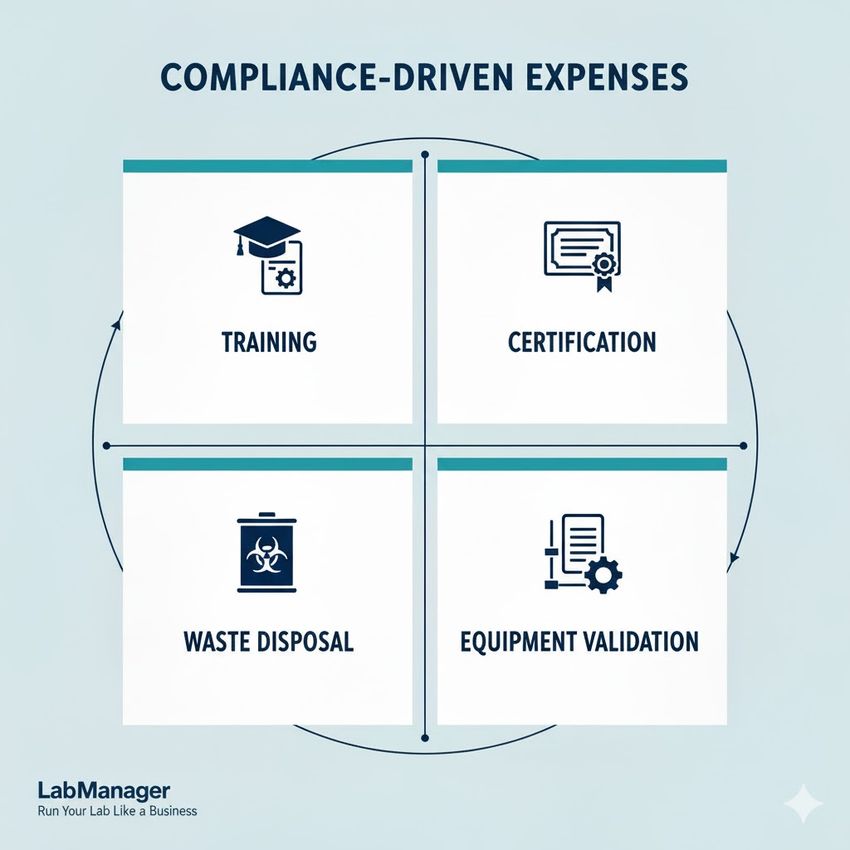
To run your lab like a business, allocate budget resources to these four non-negotiable compliance categories to avoid regulatory fines and ensure operational quality.
GEMINI (2025)
- Mandatory staff training and continuing education (e.g., IATA dangerous goods shipping, biohazard handling).
- Certification and licensing fees (e.g., CLIA certification, DEA registration for controlled substances).
- Specialized waste disposal services for biohazardous, chemical, and radioactive materials.
- Scheduled calibration and validation of equipment like biosafety cabinets, incubators, and monitoring systems.
Regulatory adherence, such as compliance with FDA guidelines for GLP (Good Laboratory Practice) or institutional animal care and use committees (IACUC), introduces mandatory reporting and auditing requirements. Proactive audit preparedness involves continuously reviewing internal controls. It means maintaining an accessible document retention system for all records and ensuring transparency in all financial transactions. Integrating compliance requirements into foundational financial strategies prevents costly penalties and operational disruptions. This action safeguards the facility's reputation and its eligibility for future grants.
Cost optimization and resource allocation in biological facilities
Optimizing operational costs requires continuous analysis of workflows and supply chain management without compromising scientific quality.
Centralizing purchasing for high-volume consumables reduces waste. Negotiating long-term vendor contracts based on committed spending volumes achieves economies of scale. Implementing robust, digital inventory management systems also contributes to efficiency. Analyzing consumption patterns helps managers identify reagents nearing expiration or those infrequently used. This data informs future procurement decisions and reduces overall inventory holdings.
Resource allocation, particularly for shared, high-value capital equipment like confocal microscopes or high-throughput sequencers, necessitates fair and transparent internal billing mechanisms. Implementing utilization tracking helps managers assess instrument throughput, identify bottlenecks, and inform decisions regarding future capital investments. This tracking provides critical justification data when applying for capital equipment grants by demonstrating high existing demand. Furthermore, incorporating energy efficiency into the budgets, such as funding for preventative maintenance on ultralow-temperature freezers, yields long-term savings and contributes to operational sustainability.
The interdependence between facility budgets and external grants
The interdependence between laboratory budgets and external grants in biological facilities necessitates integrated financial strategies. A facility's operational budget provides the stable base funding for core infrastructure, baseline technical salaries, and common utility expenses. Grants, conversely, furnish the project-specific funding for experimental consumables, specialized scientific personnel, and high-impact, single-purpose research equipment. Financial managers must structure operational budgets to demonstrate cost-sharing capabilities. This action proves to potential funders that the institution commits robust budgets and resources to the research. Concurrently, managers must ensure that grant funds only cover allowable direct costs stipulated by the sponsor and are never used to supplant the facility's core operating budget. This strategic balance prevents the underutilization of institutional resources and maximizes the longevity of available research funding. Managing multiple budgets and numerous concurrent grants requires dedicated financial expertise.
Financial mastery is indispensable for sustainable biological research
Effective financial management moves beyond simple bookkeeping; it is a core component of scientific leadership. Successfully managing operational budgets and strategically securing research grants empowers biological facilities to conduct high-quality, continuous research. Adopting proactive financial strategies, maintaining rigorous compliance, and implementing continuous cost optimization ensure the facility's long-term viability. Financial stewardship drives scientific momentum. This ultimately supports the facility's mission to produce impactful, reproducible scientific advancements that rely on consistent and reliable funding. The diligent oversight of budgets and grants forms the bedrock of translational science and discovery.
Frequently asked questions
How often should a biological facility review and re-forecast its annual budget?
A full review of the annual budgets should occur quarterly. Rolling forecasts update monthly to account for variable costs such as reagent consumption and fluctuating animal model costs, ensuring adaptability.
What is the difference between direct and indirect costs in a research grant?
Direct costs are expenditures specifically identifiable with a research project, such as salaries for research assistants or the cost of specialized equipment. Indirect costs (or facilities and administrative costs) represent institutional overhead expenses like utilities and administrative support.
How does the facility maintain compliance with federal grant requirements?
The facility maintains compliance through mandatory financial monitoring, documented effort reporting for personnel charged to grants, adherence to documented purchasing policies, and continuous internal audits of all grant-funded expenditures.
What financial metrics indicate the overall health of a laboratory's budget?
Key indicators include the burn rate (the speed at which funds are expended), the variance analysis comparing budgeted versus actual costs, and the facility's utilization rates for high-value capital equipment.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.











