The modern clinical laboratory environment often involves managing sprawling clinical diagnostic networks that span multiple physical locations. Ensuring consistent quality, efficiency, and regulatory compliance across these sites presents a formidable challenge for laboratory leaders. Operational excellence requires a unified approach to structure, personnel, and technology integration. Successfully integrating disparate facilities into a cohesive network optimizes resource utilization, enhances diagnostic throughput, and ultimately improves patient care outcomes across the entire system.
Standardizing operational protocols across the clinical diagnostic networks
Consistency in testing procedures ensures uniform, high-quality results regardless of the physical location.
Standardization forms the bedrock of reliable multisite operations. Establishing common procedures minimizes operational variability and simplifies the training of new personnel. This strategy requires the network to unify Standard Operating Procedures (SOPs) for the pre-analytical, analytical, and post-analytical phases of testing. The network must also adopt shared processes for instrument validation, calibration schedules, and preventive maintenance. Harmonizing these core technical aspects eliminates site-specific interpretation of quality metrics and significantly strengthens overall network integrity.
Furthermore, standardization must extend to personnel competency. A central authority establishes uniform proficiency testing requirements and mandatory, network-wide training modules. Personnel rotating between sites should encounter the same workflows and documentation standards, which enhances operational flexibility and redundancy within the clinical diagnostic networks.
Key areas for operational unification:
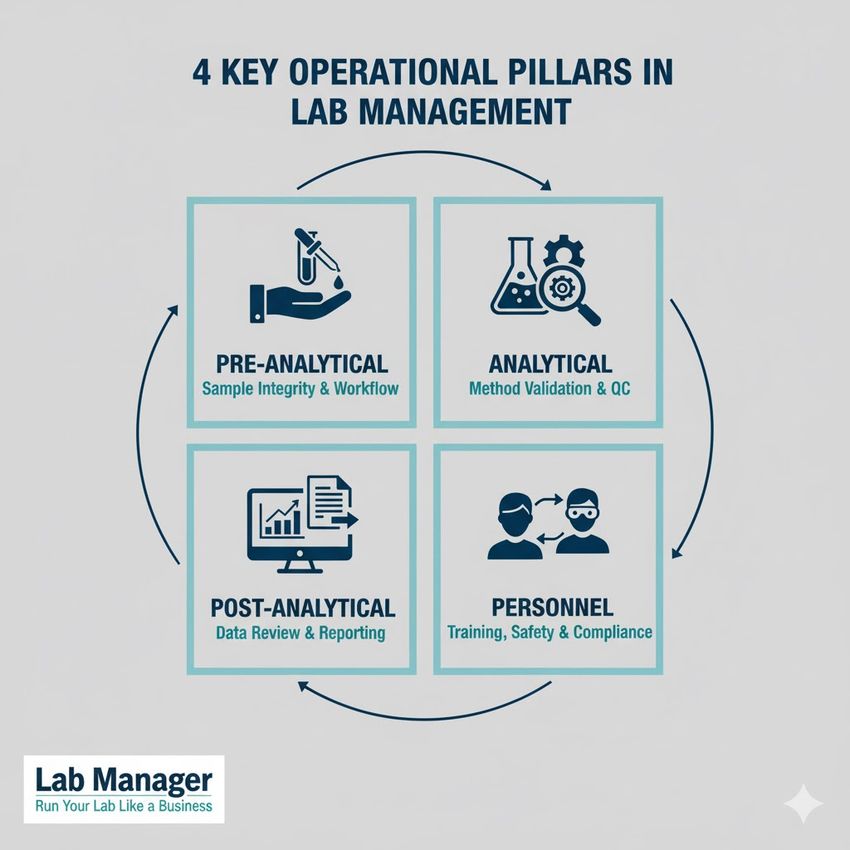
Control is key. From sample workflow to personnel compliance, these are the 4 foundational pillars required to run your lab like a business.
GEMINI (2025)
- Pre-analytical: Implementation of unified patient identification, sample collection, and sample processing protocols.
- Analytical: Mandatory use of identical reagents, instrument platforms, and quality control materials for all sites performing the same assays.
- Post-analytical: Establishment of standardized reporting formats, reference intervals, and critical value notification pathways.
- Personnel: Centralized management of competency assessments and continuing education requirements.
The World Health Organization (WHO) outlines core principles for laboratory quality management systems, specifically emphasizing standardization as a critical element for quality assurance in extensive clinical diagnostic networks. This framework provides a model for applying standardized processes consistently across geographically distributed facilities.
Centralizing information technology and data management
A robust, centralized Laboratory Information System (LIS) acts as the backbone, enabling seamless data flow and consolidated oversight for all sites.
Managing data flow across large clinical diagnostic networks requires the deployment of a single, powerful LIS instance or a tightly integrated system of component applications. Centralization ensures that all patient results, quality control data, and operational metrics reside in a unified, accessible database. This structure permits real-time visibility into performance across all laboratories, facilitating rapid problem identification and trend analysis by central leadership. A unified LIS also simplifies the management of interfaces with Electronic Health Records (EHRs), provider portals, and billing platforms, reducing complexity and the risk of data mismatch during transmission.
Data security and business continuity represents paramount concerns for any multisite structure. The centralized IT architecture must incorporate robust cybersecurity measures, including encrypted data transmission, multi-factor authentication for access, and regular vulnerability audits. For U.S.-based operations, ensuring compliance with the Health Insurance Portability and Accountability Act (HIPAA) is mandatory for protecting patient health information (PHI). Furthermore, the network requires a comprehensive disaster recovery and back-up delivery plans. This plan should specify data backup frequency, define failover mechanisms for key systems, and outline procedures for maintaining essential diagnostic services during unexpected outages that affect any single site within the clinical diagnostic networks. Adherence to Health Level Seven International (HL7) standards guides the secure and standardized exchange of clinical information within these extensive systems.
Establishing a robust quality management system and governance structure
Effective quality oversight necessitates a centralized governance model that enforces compliance and continuous improvement across every network facility.
A Quality Management System (QMS) must transcend the boundaries of individual laboratories, ensuring consistent excellence. Leadership establishes a central quality assurance team responsible for policy creation, compliance auditing, and corrective action oversight for the entire clinical diagnostic networks. This team enforces mandatory participation in unified internal quality control protocols and external quality assessment schemes (EQAS/PT), managing CLIA regulatory compliance and documentation for accreditation (e.g., CAP) on a system-wide basis.
A strong governance structure defines clear lines of authority for decision-making. Changes to protocols, assay methodologies, or LIS configurations must undergo formal review by cross-functional committees before being deployed across the network. This prevents siloed, site-specific decisions that could compromise network harmony. Key performance indicators (KPIs), such as turnaround time, assay failure rates, and critical error rates, are tracked centrally and used to drive continuous process improvement efforts. The Clinical and Laboratory Standards Institute (CLSI) publishes guidelines detailing the components of an effective QMS applicable to complex, multisite laboratory operations, which serve as an essential reference for defining these KPIs and audit cycles.
Optimizing specimen logistics and supply chain efficiency
Efficient logistical planning ensures timely sample delivery and resource availability, which are vital components of network functionality.
Efficient specimen transport is paramount, especially when core testing or specialized assays are consolidated at a centralized hub facility. Transport logistics require validation of temperature control, transit time, and stability protocols for various sample types. The transport model must account for validated courier routes, secure chain-of-custody documentation, back-up delivery plans, and continuous temperature monitoring systems to maintain sample integrity throughout the process.
Centralization of procurement and the supply chain offers substantial operational and financial benefits. Negotiating contracts and managing inventory from a single point of control reduces purchasing costs through volume discounts, minimizes the risk of stock-outs or overstocking, and ensures all sites use approved, identical consumables. This unity in supply chain management further supports the standardization efforts essential for the reliability of the clinical diagnostic networks. The system requires centralized inventory management software that tracks consumption and reorder points across all affiliated sites, maintaining optimal stock levels with minimal wastage.
Integrating molecular diagnostics into established clinical diagnostic networks requires careful strategic planning. Molecular testing often demands specialized equipment, highly trained personnel, and strict controls to prevent contamination. When deciding on the distribution of molecular assays, leadership must evaluate the trade-off between centralizing complex, high-volume testing at a core lab for efficiency versus placing urgent, low-volume tests (like point-of-care infectious disease assays) at affiliate sites for rapid turnaround. Successful integration depends heavily on standardizing nucleic acid extraction methods, thermal cycler maintenance, and validation procedures across all participating locations to ensure results remain equivalent and reliable throughout the clinical diagnostic networks. This strategic approach minimizes variability and supports the scalability of cutting-edge diagnostic technology.
Future-proofing complex clinical diagnostic networks
Effective management of clinical diagnostic networks demands a consistent, systematic approach rooted in standardization, centralized data governance, and robust quality oversight. By unifying operational protocols, consolidating information systems, and implementing a centralized governance model, laboratory operations achieve greater efficiency, minimize variance, and ensure comprehensive regulatory compliance. This cohesive strategy allows the entire network to scale new technologies, manage costs effectively, and ultimately deliver reliable, high-quality diagnostic services consistently across diverse geographic areas. Strategic investment in harmonizing process and technology secures the long-term integrity of the network.
Frequently asked questions
What is the primary operational risk in multisite clinical diagnostic networks?
The primary operational risk involves procedural variance. Inconsistent SOPs across sites lead to result discrepancies, which compromise the reliability and integrity of patient data. Centralizing SOP management mitigates this risk.
How does centralizing the LIS benefit quality control for the network?
Centralization permits real-time, consolidated quality control monitoring. It enables immediate trend analysis across all sites, allowing a central quality team to spot systemic issues faster than relying on individual site reporting structures.
Should all testing be centralized in a large clinical diagnostic network?
Not necessarily. While centralizing high-volume, complex, or expensive testing (like molecular diagnostics) is often efficient, affiliate laboratories retain responsibility for urgent testing, low-volume esoteric tests, or tests with stringent turnaround time requirements.
What is the importance of a unified supply chain within the clinical diagnostic networks?
A unified supply chain ensures that all affiliated sites utilize the same approved reagents and consumables. This consistency is essential for maintaining the standardization of analytical methods, which directly supports the quality goals of the entire network.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.