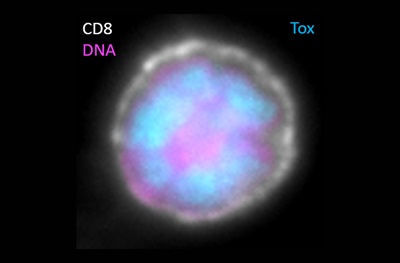
 Exhausted CD8 T cell with Tox functioning in the nucleus. (White is CD8 staining at the cell surface, pink is DNA in the nucleus, blue is Tox in the nucleus.)Credit: John Wherry, Penn MedicinePHILADELPHIA – The human immune system relies on a delicate balance of finely tuned cell types that keep germs and cancerous cells in check. In cancer and chronic infections this balance can be disrupted, resulting in immune system dysfunction or “exhaustion.” An important protein called TOX, which varies in amount in different immune cell types, controls the identity of the cells that become exhausted, according to researchers in the Perelman School of Medicine at the University of Pennsylvania. With this knowledge, investigators now have a way to accurately identify immune cells that are exhausted in a tumor or site of an infection, which could allow clinicians to improve the effectiveness of patients’ immune response to cancer treatments by reinvigorating exhausted T cells. This work is published this week in Nature.
Exhausted CD8 T cell with Tox functioning in the nucleus. (White is CD8 staining at the cell surface, pink is DNA in the nucleus, blue is Tox in the nucleus.)Credit: John Wherry, Penn MedicinePHILADELPHIA – The human immune system relies on a delicate balance of finely tuned cell types that keep germs and cancerous cells in check. In cancer and chronic infections this balance can be disrupted, resulting in immune system dysfunction or “exhaustion.” An important protein called TOX, which varies in amount in different immune cell types, controls the identity of the cells that become exhausted, according to researchers in the Perelman School of Medicine at the University of Pennsylvania. With this knowledge, investigators now have a way to accurately identify immune cells that are exhausted in a tumor or site of an infection, which could allow clinicians to improve the effectiveness of patients’ immune response to cancer treatments by reinvigorating exhausted T cells. This work is published this week in Nature.
“The discovery of TOX as the key regulator of exhausted T cells now allows us to envision immunotherapies that target, or engineer, TOX to reverse or prevent exhaustion and improve immunity to infections or cancer,” said senior author E. John Wherry, PhD, chair of the department of Pharmacology and director of the Penn Institute of Immunology.
The T cells the team studied come in three varieties and rely on efficient and coordinated transitions between different identities. Following initial activation by specific proteins, immature T cells replicate and undergo an orchestrated program of molecular rewiring to become effector T cells (TEFF), which produce inflammatory cytokines that kill offending cancer and germ cells.
If an infection or tumor is cleared, most of the TEFF pool dies, but a subset persists. This set undergoes more rewiring and forms long-lived, self-renewing memory T cells (TMEM) capable of mounting a rapid recall response should an invader be detected a second time. However, during chronic infections or with cancer, when T cell stimulation is drawn out, this program of T cell differentiation is diverted and the cells becoming ineffective against the tumor or infection—instead, they become exhausted. But, these exhausted T cells (TEX) are not totally useless. In fact, they may keep low-level germ or tumor presence in the body in check.
In this battle, Wherry likens TEX to an infantry that performs the day in and day out work of containing minor assaults, such as long-term infection by the herpes virus. On the other end of the spectrum, TEFFs are like calling in the Navy SEALs.
“They get the job of containment done, and quickly, by whipping up a cytokine storm, but there is the collateral damage of an overactive inflammatory response,” Wherry said. TEX are not strong enough to cause an increased inflammatory response, and in some cases, may strike a necessary balance between partially containing an infection or tumor without causing excessive damage to the host.
The longer TOX is expressed in a T cell the more permanent the TEX identity becomes. The level of TOX in a T cell dictates how an infection or tumor is contained by controlling the number of TEFF versus TEX cells. High and sustained induction of TOX results in the permanent existence of TEX, but the consequences of a restrained ability to fight invaders can be the persistence or progression of disease.
The team also showed that TOX shapes cell identity by making the spools on which genes are wound in the nucleus more or less available to be translated into proteins. This ability of TOX to shape the structure of a cell’s genome via its epigenome also provides insight into why changing TEX into TEFF has been difficult with other therapies. Epigenetic changes help “lock” cells into their permanent identity, but these new findings may allow researchers to change that for future immunotherapies.
This work was funded by the National Institutes of Health (AI105343, AI082630, AI115712, CA210944, AI117950, AI108545) and the Parker Institute for Cancer Immunotherapy. Wherry has a patent licensing agreement on the PD-1 pathway and is a founder of Arsenal Biosciences.
Additional Penn authors include first author Omar Khan as well as Ravi Amaravadi, Xiaowei Xu, Giorgos C. Karakousis, Tara C. Gangadhar, Lynn M. Schuchter, Jonathan Kaye, and Shelley L. Berger.













