Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) are foundational characterization techniques in modern polymer and battery R&D workflows. These thermal analysis methods provide laboratory professionals with quantitative data regarding thermal stability, composition, and phase transitions, which are essential for material selection and failure analysis. By measuring changes in mass and heat flow as a function of temperature, researchers can predict product performance under real-world operating conditions.
Distinct roles of TGA and DSC in polymer and battery R&D
TGA and DSC provide complementary data sets that define the thermal lifecycle of a material from synthesis to degradation. TGA measures the change in mass of a sample as it is heated, cooled, or held at a constant temperature, directly quantifying thermal stability and compositional content. Conversely, DSC measures the amount of energy absorbed or released by a sample, identifying phase transitions such as melting, crystallization, and glass transitions.
Laboratory professionals utilize TGA primarily to determine decomposition temperatures and volatile content. This data helps establish the upper temperature limits for processing and end-use applications. For example, TGA curves reveal the exact point of thermal onset for degradation, allowing formulators to adjust stabilizer packages in plastics or electrolytes in batteries.
DSC data is indispensable for understanding physical transformations that do not involve mass loss. It identifies the glass transition temperature (Tg), which dictates whether a polymer behaves as a rigid solid or a flexible rubber at service temperature. This technique also quantifies the degree of crystallinity, a factor that directly influences mechanical strength and chemical resistance in semi-crystalline polymers.
The combination of these techniques ensures a comprehensive understanding of material behavior. While TGA identifies how much of a material remains after thermal stress, DSC explains how the material's internal structure changes before failure occurs. Together, they form a robust framework for quality control and new product development in industrial laboratories.
Optimizing polymer formulations using TGA and DSC
Polymer R&D relies heavily on thermal analysis to tailor mechanical properties and verify composition against specifications. TGA, DSC, polymer, battery, and R&D professionals use these tools to screen raw materials, ensuring that incoming resins meet the necessary thermal criteria before manufacturing begins. This screening process prevents costly production failures caused by inconsistent feedstock or contamination.
Thermogravimetric analysis is frequently used to quantify filler content in composite materials. By heating a polymer composite in an inert atmosphere followed by an oxidative atmosphere, analysts can separate the polymer matrix from carbon black and inorganic fillers. This compositional analysis is crucial for verifying that reinforced plastics contain the correct ratio of additives to achieve desired strength and weight characteristics.
Differential scanning calorimetry is vital for optimizing curing processes in thermosets and elastomers by measuring both the heat and degree of cure. This data allows engineers to design efficient heating cycles for molding operations, ensuring that parts are fully crosslinked without wasting energy on excessive cycle times.
Thermal history analysis via DSC helps troubleshoot processing issues in manufactured parts. By performing a "heat-cool-heat" cycle, researchers can erase the thermal history imparted during manufacturing to compare the intrinsic properties of the material against the processed part. This comparison often reveals hidden issues, such as internal stress or incomplete crystallization caused by improper cooling rates.
For materials science labs and QA/QC teams, characterizing thermal properties is essential for predicting how a polymer will process and perform in the real world.
GEMINI (2026)
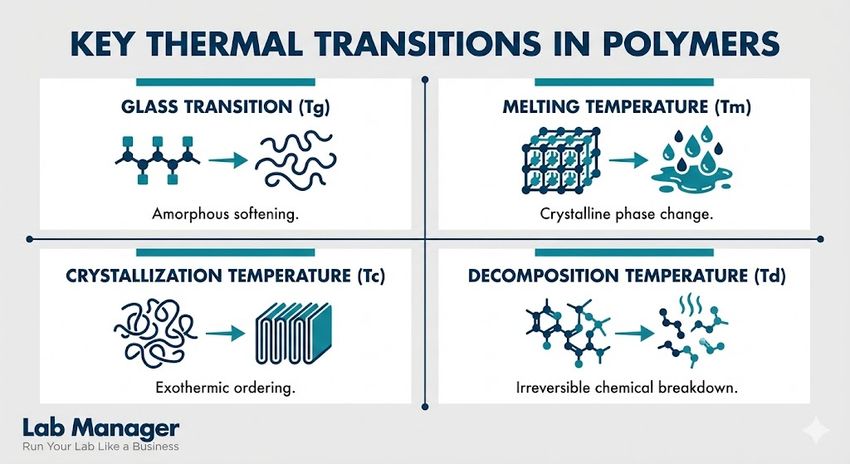
Key thermal transitions in polymers
- Glass Transition (Tg): The reversible transition in amorphous materials from a hard, brittle state to a molten or rubbery state.
- Melting Temperature (Tm): The temperature at which crystalline domains lose their structure and the polymer becomes a fluid.
- Crystallization Temperature (Tc): The temperature at which polymer chains align into an ordered structure upon cooling.
- Decomposition Temperature (Td): The point at which chemical bonds break, resulting in mass loss and material failure.
Enhancing battery safety and performance with thermal analysis
Battery R&D utilizes thermal analysis to mitigate thermal runaway risks and enhance energy density in lithium-ion and solid-state cells. In the high-stakes environment of TGA, DSC, polymer, battery, and R&D, identifying the precise thermal limits of cell components is a safety imperative. Researchers must characterize cathodes, anodes, separators, and electrolytes to ensure they remain stable under extreme thermal loads.
TGA is essential for analyzing the thermal stability of electrode materials and solid-electrolyte interphase (SEI) layers. When researchers subject cathode materials to programmed heating, they can detect weight changes associated with oxygen release—a critical precursor to thermal runaway. Accurately quantifying the temperature of oxygen evolution facilitates the selection of safer cathode chemistries for electric vehicles and consumer electronics.
DSC plays a critical role in evaluating the thermal stability of liquid electrolytes and separators. For instance, DSC detects exothermic reactions indicating electrolyte decomposition, which can generate dangerous heat and pressure within a sealed cell. Furthermore, testing separator integrity via DSC ensures the polymer membrane resists melting or shrinking at operating temperatures, preventing internal short circuits.
Analyzing binders and active material ratios represents another key application of TGA in battery research. TGA curves accurately determine the percentage of binder, conductive carbon, and active material in an electrode coating, verifying that the slurry was mixed correctly. This confirmation ensures the final coating meets electrochemical performance targets without manufacturing defects.
Thermal runaway simulation often begins with micro-calorimetry data derived from DSC. By measuring the onset temperature and the magnitude of exothermic decomposition reactions, safety engineers can model the potential energy release of a full-scale battery pack. This data informs the design of thermal management systems and safety vents required for regulatory compliance.
Benefits of Simultaneous Thermal Analysis (STA) for laboratory R&D
Simultaneous Thermal Analysis (STA) combines TGA and DSC into a single measurement, maximizing efficiency for R&D workflows. STA instruments measure both heat flow and weight change on the same sample at the same time, eliminating experimental variables associated with running two separate tests. This simultaneity ensures that phase transitions and decomposition events are perfectly correlated on the time and temperature axes.
The primary advantage of STA is the precise differentiation between thermal events that look similar but have different origins. For example, a DSC endotherm could indicate melting (no mass loss) or decomposition (mass loss). By overlaying the TGA signal, an analyst can immediately confirm whether the thermal event involves a change in mass, resolving ambiguity without a second experiment.
STA is particularly valuable for complex formulations where multiple volatile components evolve as the material undergoes structural changes. In pharmaceutical and polymer applications, identifying the exact temperature where solvent evaporation (mass loss) overlaps with a melting transition (phase change) is critical. The combined data stream allows for the kinetic analysis of complex reaction mechanisms that would be impossible to decouple using standalone instruments.
Laboratory throughput is significantly increased when employing STA for routine screening. Running a single STA experiment cuts instrument time and sample preparation time in half compared to running separate TGA and DSC protocols. This efficiency is vital for high-volume R&D environments where rapid iteration of material formulations is necessary to meet time-to-market deadlines.
Maintaining data integrity and compliance in thermal analysis
Adherence to established ASTM and ISO standards is non-negotiable for R&D data validation. Regulatory bodies require that thermal analysis methods be reproducible, calibrated, and documented according to strict protocols. Standardized testing methods ensure that data generated in one laboratory can be compared directly with data from suppliers or customers globally.
Calibration of TGA and DSC instruments is the first step in ensuring data integrity. Instruments must be calibrated using reference materials with known melting points and heat capacities, such as indium or zinc. Regular calibration checks verify that the temperature and heat flow signals remain within the specified tolerance limits required by standards like ASTM E967 (temperature calibration) and ASTM E968 (heat flow calibration).
Specific standards govern the application of thermal analysis to polymers and batteries. ASTM E1131 serves as the standard test method for compositional analysis by thermogravimetry, widely used for quality control. For DSC, the ISO 11357 series provides the framework for determining melting and crystallization temperatures, ensuring consistency across the plastics industry.
Data traceability is increasingly important in regulated industries like automotive and medical device manufacturing. Modern thermal analysis software includes audit trails that record every user action, method modification, and data calculation. These electronic records are essential for demonstrating compliance with FDA 21 CFR Part 11 and other quality management systems.
Common Standards in Thermal Analysis
Standard | Description | Application |
|---|---|---|
ASTM E1131 | Standard Test Method for Compositional Analysis by Thermogravimetry | General Composition |
ASTM D3418 | Transition Temperatures and Enthalpies of Fusion/Crystallization of Polymers by DSC | Polymers |
ISO 11357 | Plastics – Differential Scanning Calorimetry (DSC) | Plastics R&D |
ASTM E1269 | Standard Test Method for Determining Specific Heat Capacity by DSC | Thermal Management |
The future of TGA and DSC in polymer and battery innovation
The integration of TGA and DSC into research workflows is essential for advancing material science in the polymer and battery sectors. By providing precise data on thermal stability, phase transitions, and composition, these tools allow R&D professionals to push the boundaries of performance and safety. As material formulations become more complex, the reliance on accurate, standards-compliant thermal analysis will only grow, cementing its status as a cornerstone of industrial innovation.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.













