In the fast-paced world of laboratory science, the demand for analytical methods that are not only accurate but also robust and efficient has never been greater. The traditional approach to method development—often referred to as "one-factor-at-a-time" (OFAT)—involves changing one variable while holding all others constant. While seemingly logical, this approach is both inefficient and, critically, fails to identify interactions between different factors. This can lead to methods that are fragile, difficult to transfer, and prone to failure when faced with minor variations in the lab environment.
Design of Experiments (DoE) offers a powerful alternative. It is a structured, statistical approach that allows scientists to simultaneously investigate the effects of multiple factors and their interactions on a given outcome. By moving from a trial-and-error mentality to a data-driven strategy, DoE enables the creation of more robust and reliable analytical methods in a fraction of the time. This article will serve as a guide for laboratory professionals, detailing the core principles of DoE, outlining its practical application in method development, and demonstrating how this statistical tool can transform your workflow and improve the quality of your methods.
What is Design of Experiments (DoE)?
Design of Experiments (DoE) is a systematic approach to planning, conducting, and analyzing controlled tests. Its primary goal is to determine the relationship between factors (input variables that can be manipulated) and responses (output variables or results). Instead of a scattergun approach, DoE provides a structured framework for changing multiple factors at once, in a controlled manner, to efficiently gather maximum information from a minimum number of experiments. This statistical methodology is rooted in the work of R.A. Fisher and has since become a cornerstone of quality improvement and optimization across various industries, including pharmaceuticals, chemistry, and manufacturing. By using a DoE, you are not just looking at the effect of A or B, but also the synergistic or antagonistic effect of A and B acting together, which is often the key to unlocking true method robustness. A successful DoE is a powerful tool for process understanding, leading to a "design space"—the multidimensional combination and interaction of input variables that have been demonstrated to provide assurance of quality.
Key Principles and Terminology of DoE
To effectively utilize Design of Experiments, it's essential to understand its core components. The power of DoE lies in its ability to reveal complex relationships that are impossible to detect using traditional methods.
- Factors and Levels: Factors are the independent variables that you can control and change during the experiment (e.g., column temperature, pH of the mobile phase, flow rate). Each factor is tested at different "levels"—the specific settings or values for that factor. For a simple two-level design, you might test a factor at a low setting and a high setting (e.g., Column Temperature at 25°C and 40°C).
- Responses: The responses are the dependent variables—the results you are measuring (e.g., peak area, peak tailing, retention time, separation efficiency). The goal of the experiment is to understand how changes in the factors affect the responses.
- Interactions: This is where DoE truly shines. An interaction occurs when the effect of one factor on the response depends on the level of another factor. For example, a change in flow rate might have a different effect on peak shape at a low temperature versus a high temperature. DoE systematically uncovers these interactions, which are often the hidden causes of method instability.
- Main Effects: The main effect of a factor is the average change in the response caused by changing that factor's level. DoE allows you to see the individual impact of each factor, while simultaneously studying their combined effects.
By defining these elements upfront, a DoE provides a clear and organized roadmap for your experiments, ensuring that you gather the most meaningful data possible. It is this structured approach that distinguishes DoE from traditional methods and makes it a cornerstone of modern analytical method development.
Common DoE Designs for Analytical Method Development
Selecting the right DoE design is a critical step that depends on the number of factors you wish to screen and the complexity of the interactions you are investigating. There are several common designs, each with its own advantages.
- Factorial Designs: The gold standard for investigating a small number of factors. A full factorial design tests every possible combination of factor levels. For instance, a 23 design tests three factors, each at two levels, requiring 2×2×2=8 experiments. While powerful for uncovering all main effects and interactions, the number of experiments grows exponentially, making it impractical for more than a handful of factors.
- Fractional Factorial Designs: A more efficient alternative to full factorial designs when you have many factors. A fractional factorial design tests a select fraction of all possible combinations. For example, a 27−4 design tests 7 factors in only 8 experiments. These designs are ideal for screening a large number of factors to quickly identify the few that have a significant impact on the response.
- Plackett-Burman Designs: Another excellent screening design for a large number of factors. Plackett-Burman designs are highly efficient but are typically used to identify the most significant factors, not to study complex interactions.
- Response Surface Methodology (RSM): Once you have identified the key factors, RSM designs are used to model and optimize the response. They are often used to find the "sweet spot" or the optimal combination of factor levels that maximizes or minimizes a particular response. Common RSM designs include Central Composite and Box-Behnken designs.
Each of these designs is suited for a different stage of method development. A typical workflow might start with a fractional factorial design to screen for the most important factors, followed by an RSM design to optimize the levels of those critical factors.
The DoE Workflow in Practice: A Step-by-Step Guide
Implementing a Design of Experiments workflow is a disciplined process that, when followed correctly, leads to efficient and successful outcomes.
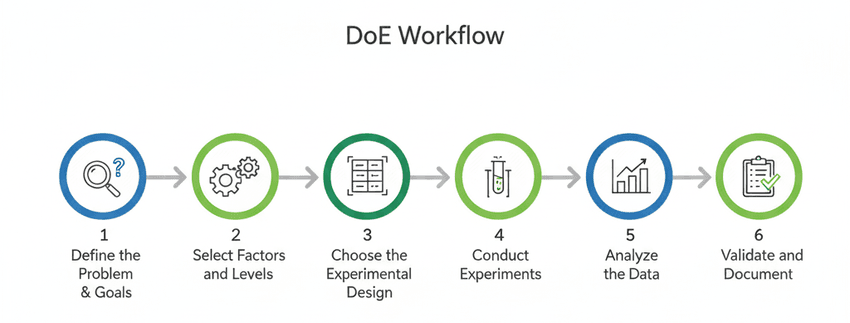
The DoE workflow follows these six steps.
GEMINI (2025)
Define the Problem and Goals: Clearly state the objective of your experiment. What analytical method are you developing? What are the key performance indicators (responses) you want to optimize, such as resolution, peak shape, or sensitivity? This foundational step ensures everyone is aligned and focused on the same goal.
Select Factors and Levels: Identify all the variables that could potentially influence your responses. These are your factors. For each factor, determine the range or "levels" you want to test. This requires a strong understanding of the analytical chemistry of the method.
Choose the Experimental Design: Based on the number of factors and the stage of your method development, select an appropriate DoE design. Use a screening design for a large number of factors and an optimization design for a smaller number of factors.
Conduct the Experiments: Carefully execute the experiments according to the randomized run order generated by the DoE software. Randomization is key to minimizing the influence of uncontrolled, lurking variables.
Analyze the Data: Once all experiments are complete, input the results into a statistical software package. The software will generate statistical models and plots that show the main effects and interactions of your factors on the responses. This analysis will clearly identify the critical process parameters.
Validate and Document: Based on the model, perform confirmatory experiments to validate the predicted optimal conditions. Finally, fully document your findings, including the DoE matrix, the statistical analysis, and the final optimized method parameters. This documentation is crucial for regulatory submissions.
The Clear Advantages of a DoE-Based Approach
Moving from a one-factor-at-a-time (OFAT) approach to Design of Experiments offers profound benefits that extend beyond a single experiment.
Efficiency: DoE significantly reduces the number of experiments required to obtain a comprehensive understanding of a method. Instead of performing dozens or even hundreds of individual runs, a well-designed DoE can provide a wealth of information from a small, manageable number of experiments. This saves valuable time, reagents, and resources, leading to a faster method development cycle.
Robustness and Quality: By systematically uncovering factor interactions, DoE helps you create methods that are inherently more robust. You can identify and define a method's "design space"—the set of operating conditions that consistently produce a high-quality result. This means your method will be less susceptible to minor changes in the lab, from variations in ambient temperature to differences between instrument models. A robust method is a reliable method, which is a key goal for any laboratory.
Process Understanding: DoE provides deep insight into the underlying chemistry and physics of your analytical procedure. It allows you to model how changes in one factor influence the effect of another, giving you a predictive capability that is impossible with OFAT. This deeper understanding is a regulatory requirement under quality by design (QbD) principles and is essential for troubleshooting and continuous improvement.
Regulatory Compliance: Regulatory bodies, such as the FDA, have increasingly emphasized the principles of Quality by Design (QbD). The use of DoE is a cornerstone of a QbD approach, as it demonstrates a thorough understanding of the method and its critical parameters. By using DoE, laboratories can build a strong scientific case for their methods, which can streamline the regulatory submission and approval process.
Embracing DoE for Future-Proof Method Development
The landscape of analytical science is evolving, and the expectations for method performance and understanding are higher than ever. The old way of developing methods, through trial and error, is no longer sufficient to meet the demands of speed, quality, and regulatory compliance. Design of Experiments is more than just a statistical tool; it is a paradigm shift that enables laboratory professionals to be more efficient, more knowledgeable, and more effective.
By adopting a DoE-based approach, you move from simply developing a method to truly understanding it. You replace guesswork with data-driven decisions and replace fragile methods with robust, reliable, and "future-proof" ones. This not only enhances the quality of your work but also positions your laboratory at the forefront of modern scientific practice. The investment in learning and applying DoE principles is an investment in the long-term success and integrity of your analytical procedures.
Frequently Asked Questions About Design of Experiments
Is DoE only for complex analytical methods?
No. While it is highly beneficial for complex methods, DoE can be applied to any method, from simple dissolution testing to complex chromatography. The principles of identifying and optimizing factors apply universally.
Do I need expensive software to perform DoE?
While specialized software makes the process easier, the basic principles can be understood and applied with standard statistical packages. The key is the structured thought process, not the specific software.
How do you choose which factors to include in a DoE?
You should include any variable that you believe could influence the method's performance. This can be based on prior knowledge, experience, or preliminary screening experiments. It is better to include a factor and find it is not significant than to miss a critical one.
What is the main difference between DoE and one-factor-at-a-time (OFAT) experiments?
The main difference is that DoE changes multiple factors simultaneously and systematically, allowing it to detect interactions between factors. OFAT changes only one factor at a time, making it unable to identify these crucial interactions.














