Liquid chromatography-mass spectrometry (LC-MS) data analysis frequently represents the most significant bottleneck in modern laboratory workflows, often consuming more time than sample preparation and acquisition combined. By implementing automated post-acquisition strategies, laboratories can significantly reduce the risk of manual transcription errors and standardization variability that plague high-throughput environments.
Transitioning from manual peak integration to automated processing allows scientists to focus on data interpretation rather than routine manipulation. This shift not only accelerates turnaround times but also strengthens compliance with data integrity guidelines.
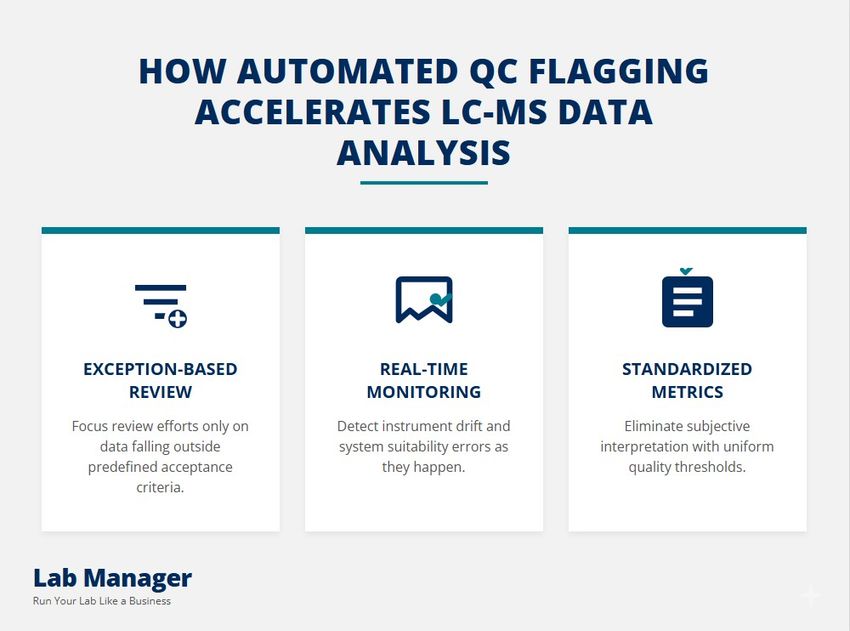
How automated QC flagging accelerates LC-MS data analysis
Automated quality control (QC) flagging systems drastically decrease the time required for data review by instantly identifying samples that fall outside pre-defined acceptance criteria. Instead of manually inspecting every chromatogram, analysts can rely on software algorithms to evaluate system suitability parameters such as retention time stability, peak asymmetry, and mass accuracy drift.
Manual review of LC-MS data is often the biggest bottleneck in the modern analytical lab. By shifting to automated QC flagging, labs can move from reactive troubleshooting to proactive management.
GEMINI (2026)
- Exception-Based Review: Automation enables "review by exception," where only flagged data requires human intervention, reducing the review workload significantly in some workflows.
- Real-Time Monitoring: Advanced systems monitor instrument performance in real-time, triggering automatic re-injections or pause sequences if QC standards fail.
- Standardized Metrics: Algorithms apply consistent integration parameters across large datasets, eliminating the inter-operator variability inherent in manual peak picking.
Routine reliance on manual QC checks often leads to "alert fatigue" and oversight; however, automated systems provide continuous, consistent monitoring. According to recent industry reports, laboratories implementing automated system suitability testing (SST) experience fewer batch failures and reduced instrument downtime. Regulatory bodies like the FDA emphasize that automated checks must be validated to ensure they reliably detect substandard data without masking genuine analytical issues.
Why cloud-based storage is essential for LC-MS data analysis
Cloud-based data storage provides the scalable infrastructure necessary to handle the massive datasets generated by modern high-resolution LC-MS instruments without the limitations of on-premises servers. Moving data to the cloud decouples data acquisition from analysis, allowing researchers to process heavy files from any location using high-performance computing resources.
- Scalability: Cloud platforms automatically adjust storage capacity to accommodate fluctuating data volumes, preventing the need for costly hardware upgrades.
- Disaster Recovery: Automated backups and redundant storage locations ensure data is protected against local hardware failures or site-specific disasters.
- Collaboration: Centralized cloud repositories facilitate secure data sharing between departments or external partners without the security risks of transferring physical media.
The adoption of cloud-native platforms supports the use of vendor-neutral data formats (such as mzML), which improves interoperability between different instrument manufacturers. Security remains a primary concern; however, reputable cloud providers offer encryption at rest and in transit that often exceeds the capabilities of local laboratory networks. By leveraging the cloud, laboratories can implement automated data lifecycle management policies that archive older projects automatically, keeping active storage optimized for current work.
How LIMS integration optimizes LC-MS post-acquisition workflows
Integrating a Laboratory Information Management System (LIMS) with LC-MS software creates a seamless, bidirectional flow of information that eliminates manual data entry and ensures sample traceability. This integration allows the LIMS to automatically push sample worklists to the instrument and pull results back immediately after processing, closing the data loop.
Feature | Benefit |
|---|---|
Bidirectional Communication | Eliminates manual transcription errors and ensures sample ID matching. |
Automated Reporting | Generates Certificates of Analysis (CoA) instantly upon data approval. |
Sample Tracking | Maintains a complete chain of custody from receipt to final result. |
A fully integrated ecosystem prevents the formation of "data silos" where results remain trapped on instrument PCs. When the LIMS acts as the central hub, it can trigger downstream workflows—such as invoicing or follow-up testing—based on the specific LC-MS results received. Furthermore, integration facilitates better resource management by tracking instrument utilization and consumable usage directly within the management system.
Achieving regulatory compliance with automated data processing
Automated data processing systems inherently support regulatory compliance by generating comprehensive, unalterable audit trails that document every step of the analysis pipeline. In regulated environments adhering to GMP or GLP standards, the ability to reconstruct exactly how a result was derived—from raw data to final report—is mandatory.
- Audit Trails: Automation ensures that all parameters, processing methods, and user actions are time-stamped and attributable, meeting 21 CFR Part 11 requirements.
- Data Integrity: Automated transfers reduce the human touchpoints where data could be accidentally or intentionally altered, supporting ALCOA+ principles.
- Validation Efficiency: Validated automated workflows reduce the need for repetitive manual verification steps, simplifying the overall validation master plan.
Regulatory agencies like the FDA and EMA increasingly scrutinize data integrity during inspections. Automated systems that enforce version control and prevent unauthorized deletion provide a higher level of assurance than hybrid paper-electronic systems. By locking down processing methods and limiting access rights through role-based permissions, laboratories can demonstrate a state of control that is difficult to achieve with manual processes.
Ensuring Data Integrity Through Automated Audit TrailsThe implementation of automated audit trails is the cornerstone of maintaining data integrity in computerized laboratory systems. An effective audit trail functions as a secure, computer-generated, time-stamped electronic record that allows for the reconstruction of the course of events relating to the creation, modification, or deletion of an electronic record. In LC-MS workflows, this means capturing not just the final integration results, but the specific processing method version, the user who applied it, and the exact date and time of the action. This continuous monitoring prevents "data testing" or orphan data, where analysts might reprocess samples multiple times until a desired result is achieved without record. By removing the ability for users to disable or manipulate these logs, laboratories ensure compliance with 21 CFR Part 11 and Annex 11 regulations, thereby protecting the scientific validity of their output.
Optimizing laboratory efficiency through LC-MS automation
Automating LC-MS data analysis effectively removes the most significant bottlenecks in the analytical pipeline, delivering faster and more reliable results. By combining automated QC flagging, cloud-based scalability, and LIMS integration, laboratories can ensure data integrity while maximizing throughput. This strategic shift allows scientific staff to focus on high-value interpretation rather than manual processing, ultimately enhancing the laboratory's operational and regulatory performance.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.














