The fields of materials science and chemical research are at a pivotal moment. The drive for innovation is now inextricably linked with the imperative of sustainability. For too long, the environmental and health impacts of chemical processes were considered secondary to performance and yield. However, a new paradigm is emerging, guided by the principles of green chemistry. This approach is not merely about minimizing harm but about proactively designing chemical products and processes to reduce or eliminate the use and generation of hazardous substances. It is a transformative framework that is essential for developing environmentally safe materials and establishing truly sustainable lab practices.
This article provides an in-depth exploration of how the core tenets of green chemistry can be applied to materials development. It will detail strategies for eco-friendly synthesis, the importance of selecting environmentally safe materials, and the application of these principles on a larger scale. By embracing this approach, laboratories can not only reduce their ecological footprint but also pioneer new methodologies that are safer, more efficient, and more innovative.
The Twelve Principles of Green Chemistry in Practice
At its core, green chemistry is guided by a set of twelve principles that provide a roadmap for designing cleaner and more efficient chemical processes. Understanding these principles is the first step toward implementing sustainable lab practices. When applied to materials development, they challenge researchers to rethink every stage of the process, from raw material selection to final product design.
The Foundational Principles
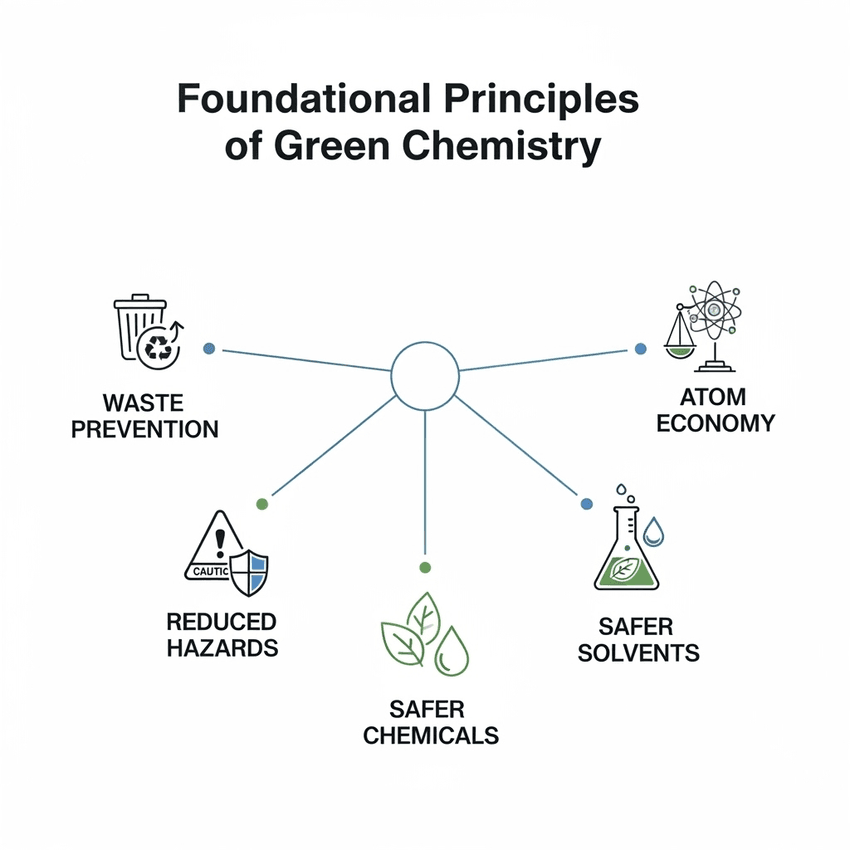
The twelve principles of green chemistry contain these five foundational principles.
GEMINI (2025)
- Waste Prevention: The most crucial principle is to prevent waste at the source. This means designing reactions that do not produce harmful byproducts, a concept often referred to as "atom economy."
- Atom Economy: A measure of how efficiently atoms from reactants are incorporated into the desired product. Reactions with high atom economy produce less waste.
- Less Hazardous Synthesis: Designing reactions that use and produce substances with little to no toxicity to human health and the environment.
- Designing Safer Chemicals: Creating chemical products that are fully effective but have low or no toxicity.
- Safer Solvents and Auxiliaries: Choosing solvents that are non-toxic and biodegradable, or, where possible, eliminating their use altogether.
The Operational Principles
- Design for Energy Efficiency: Conducting reactions at ambient temperature and pressure whenever possible to minimize energy consumption.
- Use of Renewable Feedstocks: Sourcing raw materials from renewable resources rather than depletable ones.
- Reduce Derivatives: Avoiding unnecessary steps that require additional reagents and generate more waste.
- Catalysis: Using catalytic reagents that can be used in small amounts and reused, rather than stoichiometric reagents that are consumed in the reaction.
- Design for Degradation: Creating materials that break down into innocuous products at the end of their functional life.
- Real-time Analysis for Pollution Prevention: Developing and using in-process monitoring and control to prevent the formation of hazardous substances.
- Inherently Safer Chemistry for Accident Prevention: Choosing substances and forms of substances used in a chemical process to minimize the potential for chemical accidents, including releases, explosions, and fires.
The application of these principles in materials development leads directly to cleaner, safer, and more cost-effective outcomes. It is the very essence of sustainable lab practices.
Eco-Friendly Synthesis and Green Manufacturing
The transition to green chemistry is most visible in the development of new synthetic methods. Eco-friendly synthesis is about moving away from traditional, hazardous methods toward innovative processes that are inherently more sustainable. This includes exploring new reaction conditions, catalysts, and raw materials.
One key area of innovation is catalysis. Traditional synthesis often relies on stoichiometric reagents that are consumed in the reaction and must be disposed of. By contrast, catalytic processes, particularly those involving heterogeneous catalysts, can be designed to be highly selective, operate under milder conditions, and be easily recovered and reused. This approach not only reduces waste but also improves the overall efficiency of the process.
Eco-friendly synthesis also emphasizes the use of alternative reaction media. Traditional organic solvents are often volatile and toxic. The use of alternatives like water, supercritical carbon dioxide, or ionic liquids as solvents can significantly reduce the health risks and environmental footprint of a process. Furthermore, solvent-free reactions, where reactants are mixed directly or in a molten state, represent the ultimate form of solvent-related waste reduction.
When a material developed in the lab is scaled up for production, the principles of green manufacturing become critical. Green manufacturing ensures that large-scale production adheres to the same green chemistry standards established in the lab. This includes:
- Continuous Flow Processes: Shifting from batch to continuous flow reactors to improve efficiency and safety.
- Process Intensification: Designing smaller, more efficient reactors that use less energy and materials.
- Waste Valorization: Turning waste byproducts into useful materials, which is a key tenet of the circular economy.
Designing and Selecting Environmentally Safe Materials
The principles of green chemistry do not end with the manufacturing process; they extend to the final product itself. Designing environmentally safe materials is a fundamental goal. This involves considering the entire life cycle of a material, from cradle to grave, ensuring it poses minimal risk to human health and the environment at every stage.
A primary concern is toxicity. Many common materials contain or release toxic substances, from heavy metals in electronics to volatile organic compounds (VOCs) in paints and adhesives. Green chemistry challenges researchers to find non-toxic substitutes. For example, replacing cadmium-based quantum dots with indium phosphide-based ones for display technologies or developing bio-based plastics that do not require bisphenol A (BPA) for their synthesis.
Another critical aspect of designing environmentally safe materials is considering their end-of-life. Materials should be designed to either be easily recycled or to safely degrade after their intended use. Biodegradable plastics, for instance, are designed to break down into harmless compounds in a compostable or natural environment, thereby addressing the persistent problem of plastic waste. Similarly, materials designed for easy disassembly can be more easily recycled, allowing valuable components to be recovered and reused.
The selection of environmentally safe materials also involves a focus on the source of the raw materials. Choosing materials derived from renewable feedstocks, such as plant-based polymers or agricultural byproducts, over fossil fuel-based ones is a key strategy for reducing a material's carbon footprint and its dependence on non-renewable resources.
The Broader Impact and Future of Green Chemistry
The adoption of green chemistry principles in materials development has a far-reaching impact that extends beyond a single laboratory or a single product. It is a catalyst for institutional change, fostering a new culture of innovation and responsibility.
By championing eco-friendly synthesis and sustainable lab practices, researchers can secure funding from organizations that prioritize sustainability, attract top talent who are passionate about making a difference, and enhance their institution's reputation. The economic benefits are also tangible. By reducing waste, improving efficiency, and eliminating the need for expensive and hazardous disposal, green chemistry can lead to significant cost savings. Furthermore, the development of environmentally safe materials opens up new markets and creates competitive advantages in an increasingly sustainability-conscious world.
The future of materials research lies in the integration of green chemistry principles into every aspect of the scientific process. From the initial design of a molecule to its scale-up for green manufacturing, sustainability must be a core consideration. It is a commitment that ensures scientific progress serves not only the immediate needs of society but also the long-term health of the planet.
Frequently Asked Questions (FAQs) on Green Chemistry
What is the fundamental difference between green chemistry and environmental chemistry?
Green chemistry is a proactive approach focused on designing chemical products and processes to eliminate hazards from the outset, whereas environmental chemistry is reactive, studying the effects of pollutants and existing hazards in the environment.
How can a lab transition from traditional to sustainable lab practices?
A lab can begin by conducting a process audit to identify areas of high waste and resource consumption. This can be followed by a gradual implementation of green chemistry principles, starting with the use of safer solvents and more efficient catalysts.
What role does atom economy play in eco-friendly synthesis?
Atom economy is a critical metric in eco-friendly synthesis because it measures the efficiency of a reaction. A high atom economy ensures that most atoms from the starting materials are incorporated into the final product, minimizing waste.
How can a material be considered environmentally safe?
A material can be considered an environmentally safe material if it is non-toxic, derived from renewable or abundant resources, requires minimal energy to produce, and is designed to either be easily recyclable or safely biodegradable at the end of its life.