Thermal analysis provides critical data on the physical and chemical stability of pharmaceutical compounds by measuring material properties as a function of temperature or time. Laboratory professionals utilize thermal analysis to identify drug degradation pathways, characterize polymorphs, and ensure the long-term efficacy of active pharmaceutical ingredients (APIs). These techniques are fundamental for establishing the degradation profile of a drug substance under varying environmental conditions. Quantitative data derived from thermal methods supports regulatory submissions and informs the design of robust formulations. By subjecting samples to controlled thermal stress, analysts can rapidly generate screening estimates for long-term stability, though these predictions must ultimately be confirmed with real-time data per ICH Q1A(R2).
Quantifying drug degradation with thermal analysis
Thermal analysis quantifies drug degradation by monitoring changes in physical properties such as mass, heat flow, or mechanical stiffness during heating or cooling cycles. These changes correspond to specific chemical reactions, including hydrolysis, oxidation, or thermal decomposition, which compromise the integrity of the API. Integrating these measurements allows scientists to calculate the reaction enthalpy and onset temperature of degradation events.
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) are the primary methods used to detect these instabilities. TGA measures the change in mass associated with desolvation or decomposition, providing stoichiometric data on the degradation process. DSC measures the heat energy absorbed or released, identifying phase transitions that may precede or accompany chemical breakdown.
Advanced instrumentation couples these thermal techniques with evolved gas analysis (EGA) to identify the specific volatile byproducts of degradation. This hyphenated approach provides a comprehensive chemical fingerprint of the breakdown mechanism. Understanding these pathways is essential for stabilizing formulations against thermal stress during manufacturing and storage.
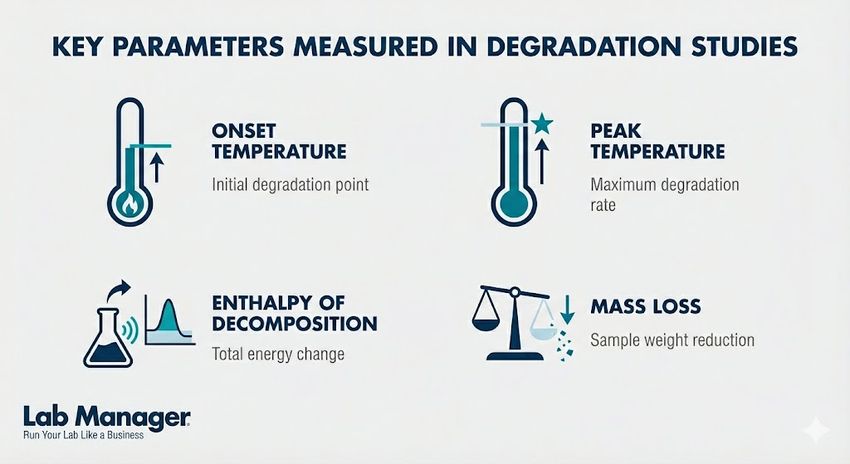
When assessing material stability and safety, precision is everything. Comprehensive degradation studies rely on capturing specific thermal and physical changes to predict product behavior under stress.
GEMINI (2026)
Key parameters measured in degradation studies
- Onset Temperature (Tonset): The temperature at which the degradation reaction becomes measurable.
- Peak Temperature (Tpeak): The point of maximum reaction rate, indicating the most rapid degradation.
- Enthalpy of Decomposition (Delta Hdec): The total energy released or absorbed during the breakdown process.
- Mass Loss (Delta m): The percentage of sample mass lost due to the release of volatiles or gaseous decomposition products.
Differential scanning calorimetry (DSC) in stability testing
Differential scanning calorimetry (DSC) identifies drug degradation by detecting the heat flow differences between a sample and a reference during a controlled temperature program. This technique is particularly sensitive to physical instability, such as polymorphic transitions, which can alter the dissolution rate and bioavailability of a drug. DSC data distinguishes between melting (an endothermic process) and decomposition (which can be exothermic or endothermic), providing a clear thermal profile of the API.
In compatibility studies, DSC is used to screen for interactions between the API and proposed excipients. A significant shift or disappearance of the API's melting peak when mixed with an excipient indicates a potential incompatibility. This early screening prevents the advancement of unstable formulations into expensive clinical trials.
Modulated DSC (mDSC) separates reversible heat flow (glass transitions, melting) from irreversible heat flow (decomposition, curing). This separation allows for a more accurate interpretation of complex overlapping thermal events. It ensures that kinetic parameters are calculated based solely on the relevant degradation capability.
Applications of DSC in stability assessment
- Purity Determination: Using the Van't Hoff equation to calculate purity based on melting point depression.
- Polymorph Screening: Identifying stable and metastable crystal forms that affect shelf life.
- Glass Transition Measurement (Tg): Determining the temperature at which amorphous solids transition to a rubbery state, affecting mobility and reactivity.
- Eutectic Impurity Detection: Identifying impurities that form low-melting mixtures with the API.
Using thermogravimetric analysis (TGA) for kinetic modeling
Thermogravimetric analysis (TGA) provides the mass-change data necessary to determine the kinetics of drug degradation reactions involving volatile components. By measuring the rate of mass loss as a function of temperature, analysts can compute the activation energy (Ea) and the pre-exponential factor (A) of the decomposition process. These kinetic parameters are inputs for the Arrhenius equation, which projects reaction rates to ambient storage temperatures.
TGA allows for the differentiation between unbound moisture, bound solvates, and chemical decomposition. The first stage of weight loss typically corresponds to dehydration or desolvation, while subsequent stages indicate the breakdown of the molecular structure. Accurate distinction between these events is crucial for establishing the dry weight basis of the API.
Dynamic TGA experiments, performed at multiple heating rates, enable the application of model-free isoconversional methods. These methods yield reliable predictions of oxidative stability and thermal lifetime without assuming a specific reaction model. This robust data supports the expiration dating of pharmaceutical products.
Common kinetic methods applied to TGA data
Method | Description | Application |
|---|---|---|
Kissinger Method | Uses peak temperatures from multiple heating rates. | Determining activation energy (Ea) without assuming reaction order. |
Flynn-Wall-Ozawa | An integral isoconversional method. | analyzing complex degradation mechanisms with varying Ea. |
Coats-Redfern | Uses data from a single heating rate curve. | Rapid estimation of kinetic parameters for simple decomposition. |
Isothermal TGA | Measures mass loss at a constant temperature over time. | Simulating long-term storage conditions and accelerated aging. |
Predicting drug shelf life using kinetic analysis
Kinetic analysis of thermal data allows laboratory professionals to extrapolate high-temperature degradation rates to predict the long-term stability of a drug at room temperature. By applying the Arrhenius equation to data obtained from TGA or DSC, analysts calculate the rate constant (k) for the degradation reaction. This calculation assumes that the reaction mechanism remains consistent across the measured temperature range.
Advanced software packages automate the processing of non-isothermal data to generate stability plots. These plots predict the time required for a drug to degrade by a specified percentage (e.g., 10%, or t90) under various storage conditions. This predictive capability significantly reduces the time required to estimate shelf life compared to traditional real-time stability studies.
However, predictions must be validated against real-time data to ensure accuracy. Changes in humidity or physical state (e.g., melting) can alter the reaction mechanism, rendering the Arrhenius extrapolation invalid. Therefore, kinetic modeling serves as a powerful screening tool that complements, rather than replaces, formal ICH stability protocols.
Regulatory standards for thermal stability and degradation data
Regulatory bodies require robust evidence of thermal analysis validation to demonstrate that drug degradation profiles are well-understood and controlled. Guidelines such as ICH Q1A(R2) mandate stress testing to identify likely degradation products and validate the stability-indicating power of analytical procedures, often complemented by ICH Q1B protocols for photostability. Thermal data supports the selection of appropriate packaging and storage conditions by defining the drug's thermal limits.
The FDA and EMA expect stability data to cover the entire physiological and environmental range the drug might encounter. This includes transport excursions and sterilization processes that involve heat. Data from thermal analysis justifies the exclusion of certain environmental factors from the label if stability is proven.
Documentation must include instrument calibration records, typically using indium or zinc standards for DSC. Method validation must demonstrate specificity, linearity, and precision for the intended thermal measurement. Adherence to ASTM E2550 or USP <891> ensures that thermal methods meet accepted industry standards for data integrity.
Regulatory documentation checklist
- Instrument Calibration: Verification of temperature and enthalpy accuracy using certified reference materials.
- Method Validation: Proof that the thermal method can detect degradation at relevant levels.
- Stress Testing Results: Data showing the intrinsic stability of the molecule under thermal stress.
- Impurity Profiling: Correlation of thermal events with specific degradation products identified by chromatography.
Ensuring pharmaceutical stability with thermal analysis
Thermal analysis is an indispensable tool for characterizing drug degradation and ensuring the safety and efficacy of pharmaceutical products. By leveraging techniques like DSC and TGA, laboratory professionals can rapidly identify stability risks, model degradation kinetics, and predict shelf life with high precision. These methods provide the scientific evidence required to select stable formulations and comply with rigorous regulatory standards defined by the FDA and ICH. Ultimately, the integration of thermal data into the development pipeline accelerates the delivery of high-quality therapeutics to the market.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.














