Pharmaceutical crystallography plays a pivotal role in the drug development lifecycle by defining the solid-state properties of active pharmaceutical ingredients (APIs) and excipients. Laboratory professionals rely on X-ray diffraction (XRD) as one of the definitive analytical techniques for identifying atomic arrangements within these materials. While complementary methods exist, XRD provides critical data that links crystal structure to physicochemical properties—such as solubility and dissolution rate—which ultimately influence bioavailability and therapeutic performance. The ability to distinguish between various solid forms distinguishes pharmaceutical crystallography as a critical step in formulation science and quality assurance. By leveraging high-resolution diffraction data, analysts ensure that the physical structure of a drug substance meets rigorous standards before reaching the patient.
Principles of x-ray diffraction in solid-state analysis
Understanding the interaction between X-rays and crystal lattices allows for precise structural characterization of pharmaceutical materials. At the atomic level, crystalline solids consist of regular, repeating arrays of atoms or molecules. When an incident X-ray beam interacts with the electron clouds surrounding these atoms, scattering occurs. Constructive interference of these scattered waves happens only when the conditions satisfy Bragg’s Law. This fundamental principle relates the wavelength of the electromagnetic radiation to the diffraction angle and the lattice spacing between atomic planes. In pharmaceutical crystallography, the resulting diffraction pattern serves as a unique fingerprint for a specific crystal structure, appearing as a series of peaks with distinct positions and intensities.
Laboratory professionals utilize this fingerprint to determine unit cell dimensions, space groups, and atomic positions with high precision. The technique distinguishes between crystalline and amorphous regions within a sample, providing essential data regarding the degree of crystallinity. Since the physical properties of a drug—such as solubility, melting point, and mechanical stability—depend heavily on its internal structure, XRD data provides the foundational knowledge required for rational drug design. Modern diffractometers employ advanced detectors, such as high-speed 1D silicon strip detectors or 2D area detectors, alongside high-brilliance sources (typically copper or molybdenum anodes). These technological advancements allow analysts to capture high-quality data even from weakly diffracting organic compounds or limited sample quantities, significantly reducing data collection times in high-throughput environments.
X-Ray Diffraction (XRD) remains a cornerstone technique for material characterization in modern laboratories.
GEMINI (2026)
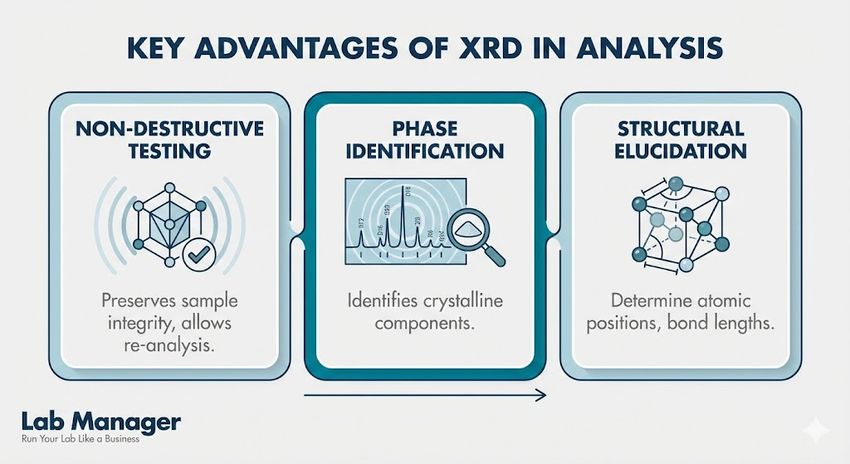
Key advantages of XRD in analysis
- Non-destructive testing: The technique analyzes samples without altering their chemical composition, allowing for subsequent testing or sample recovery.
- Phase identification: Analysts unequivocally identify crystalline phases in mixtures, including impurities, excipients, and degradation products.
- Structural elucidation: Single-crystal diffraction resolves the complete 3D molecular structure, confirming stereochemistry, absolute configuration, and bonding networks.
Polymorph screening and identification methods
Identifying distinct crystal forms ensures consistent bioavailability and stability throughout the manufacturing process and shelf life of the final product. Polymorphism, the ability of a solid material to exist in more than one crystal structure or form, represents a significant challenge and opportunity in pharmaceutical development. Different polymorphs of the same active pharmaceutical ingredient (API) often exhibit vastly different physicochemical properties. For instance, a thermodynamically metastable form may offer superior solubility and dissolution rates compared to a stable form, yet it poses a risk of conversion during storage. X-ray diffraction remains the gold standard for detecting and characterizing these forms, providing the definitive evidence required to distinguish between solvates, hydrates, and true polymorphs.
Pharmaceutical crystallography protocols involve exhaustive screening to identify all accessible polymorphs and define their thermodynamic relationships. Powder X-ray Diffraction (PXRD) serves as the primary tool for this screening because it rapidly differentiates forms based on their unique diffraction peaks. During the development phase, researchers subject the API to various solvents, temperatures, and cooling rates to induce crystallization of different forms. Analysts must also determine whether the relationship between polymorphs is enantiotropic (where the stability order changes with temperature) or monotropic (where one form is stable across the entire temperature range). The resulting patterns are compared against reference databases or calculated patterns from single-crystal data to confirm phase purity.
Regulatory bodies, including the U.S. Food and Drug Administration (FDA) and the International Council for Harmonisation (ICH), emphasize the necessity of rigorous polymorph control. Guidelines such as ICH Q6A specifically address the need to set acceptance criteria for polymorphic forms in new drug substances. Failure to control polymorphism can lead to batch-to-batch inconsistency or, in severe cases, product recall if a new, less soluble form precipitates out of the formulation.
Quantitative phase analysis and purity assessment
Advanced diffraction methods facilitate the precise quantification of crystalline mixtures and the detection of amorphous content within drug products. While qualitative identification confirms the presence of a phase, quantitative phase analysis (QPA) determines the relative amount of each phase present in a multi-component mixture. This capability becomes essential when a drug product contains multiple active ingredients or when excipients exhibit crystallinity that interferes with the API signal. Laboratory professionals frequently employ the Rietveld refinement method for this purpose. This full-pattern fitting technique models the observed diffraction pattern using calculated profiles based on crystallographic data, allowing for accurate quantification even when peaks overlap significantly. Alternatively, the internal standard method involves adding a known quantity of a pure, highly crystalline material (like corundum) to the sample to create a calibration curve for quantification.
The detection of low-level crystalline impurities constitutes another critical application. During the manufacturing of amorphous solid dispersions—formulations designed to enhance the bioavailability of poorly water-soluble drugs—the presence of trace crystallinity indicates physical instability and potential failure of the formulation strategy. XRD provides the sensitivity required to detect these trace crystalline domains, often reaching limits of detection (LOD) below 1% when using optimized instrument parameters and long scan times. Furthermore, the technique monitors batch uniformity, ensuring that the crystallization process remains robust and reproducible at an industrial scale, preventing downstream processing issues like capping or sticking during tableting.
Comparison of diffraction techniques
Feature | Powder X-ray Diffraction (PXRD) | Single Crystal X-ray Diffraction (SCXRD) |
|---|---|---|
Sample Requirement | Polycrystalline powder or bulk solid | High-quality, distinct single crystal |
Primary Output | Phase identification, purity, crystallinity | Complete 3D molecular structure |
Throughput | High (minutes per scan) | Lower (hours to days) |
Application | Routine QC, batch release, polymorph screening | Structure determination, absolute configuration |
Regulatory expectations for solid-state characterization
Guidelines mandate comprehensive characterization to ensure the identity, strength, quality, and purity of the drug product. Regulatory agencies worldwide require detailed data regarding the solid-state form of the API. This requirement stems from the direct link between crystal structure and critical quality attributes (CQAs) like dissolution and stability. Submissions to the FDA or EMA must demonstrate that the manufacturing process consistently produces the desired polymorph and that the analytical methods used, such as XRD, are validated for specificity, precision, and limit of detection. Specifically, ICH Q6A outlines decision trees for establishing specifications, guiding the laboratory professional on when to test for polymorphism and particle size.
In addition to international guidelines, laboratory professionals must adhere to pharmacopeial standards such as USP General Chapter <941> X-Ray Diffraction. This chapter provides the framework for performing qualitative and quantitative analysis, outlining best practices for sample preparation, instrument alignment, and data interpretation. Adherence to USP <941> ensures that diffraction data is generated consistently across different laboratories and instruments. Validated XRD methods serve as the primary evidence in the Chemistry, Manufacturing, and Controls (CMC) section of a New Drug Application (NDA), proving that the applicant understands and controls the physical form of the drug throughout its lifecycle.
In-situ monitoring and non-ambient diffraction
Variable temperature and humidity studies reveal stability profiles under environmental stress, providing predictive data for storage and handling. Non-ambient X-ray diffraction extends the utility of pharmaceutical crystallography beyond static measurements at room temperature. By equipping diffractometers with environmental chambers, analysts monitor structural changes in real-time as the sample undergoes heating, cooling, or exposure to varying relative humidity (RH). This dynamic analysis proves invaluable for identifying solvates and hydrates—crystal forms containing solvent or water molecules within the lattice—and observing desolvation events as they occur.
Pharmaceutical hydrates often form during wet granulation processes or storage in humid conditions. In-situ XRD experiments track the hydration and dehydration pathways, identifying the critical water activity levels at which phase transitions occur. Similarly, variable-temperature XRD (VT-XRD) determines melting points, glass transition temperatures, and polymorphic phase transition temperatures. This data aids in defining the safe operating window for manufacturing processes such as drying, milling, and compaction, where mechanical and thermal energy might otherwise induce an unwanted phase transformation. Understanding these boundaries ensures that the final product retains its intended efficacy and shelf-life stability.
Conclusion: Utilizing XRD for pharmaceutical crystallography
X-ray diffraction serves as an indispensable tool for confirming the structural integrity of pharmaceutical materials. From the initial discovery of a new chemical entity to the final quality control of the manufactured dosage form, pharmaceutical crystallography provides the structural insights necessary to predict and control drug performance. By mastering both powder and single-crystal techniques, laboratory professionals ensure that every batch meets stringent regulatory standards for safety and efficacy. As drug molecules become increasingly complex, the reliance on robust, high-resolution XRD analysis will only continue to grow, safeguarding patient health through precise solid-state characterization.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.












