Thermal analysis for shelf-life prediction provides a rapid, data-driven alternative to real-time aging studies for pharmaceuticals, foods, and polymers. By measuring physical and chemical property changes as a function of temperature, analysts can calculate degradation kinetics and forecast long-term stability with high precision. This approach allows laboratories to screen formulations quickly and identify stability risks before committing to multi-year storage tests. Standardized methods utilizing Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA) form the foundation of these predictive models.
Understanding degradation kinetics for shelf-life predictive modeling
Thermal analysis for shelf-life prediction relies on the fundamental principle that chemical reaction rates increase with temperature. Laboratory professionals use this relationship to conduct accelerated stability testing, subjecting samples to elevated temperatures to simulate long-term storage conditions in a fraction of the time. By capturing reaction rate data at multiple isotherms or heating rates, analysts can extrapolate the product's behavior at ambient storage temperatures.
The Arrhenius equation is the mathematical core of these predictions. It correlates the reaction rate constant (k) with temperature (T), allowing for the calculation of the activation energy (Ea) required for degradation to occur. A higher activation energy typically indicates a more stable material that is less sensitive to temperature fluctuations.
Calculating kinetic parameters requires precise control over experimental variables, which is achieved through several experimental approaches:
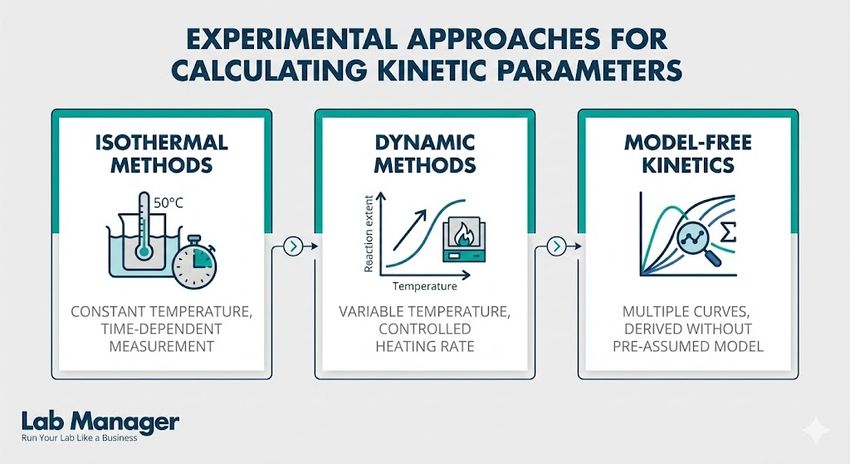
Calculating kinetic parameters requires precise control over your experimental variables. Whether you are running stability tests or characterizing new materials, the method you choose impacts the reliability of your data.
GEMINI (2026)
- Isothermal methods measure degradation at a constant temperature over time.
- Dynamic methods measure degradation while heating the sample at a constant rate.
- Model-free kinetics allow predictions without assuming a specific reaction mechanism.
Advanced kinetic analysis often utilizes isoconversional methods. These methods evaluate the activation energy as a function of conversion, revealing complex degradation pathways that single-value models might miss. This approach prevents errors associated with the "kinetic compensation effect," where changes in the pre-exponential factor mask changes in activation energy.
Applying these models enables the determination of the time required for a product to degrade to a specified limit, such as 90% potency (t90). This calculated value serves as the predicted shelf life. Accuracy in this modeling phase is critical, as minor errors in determining activation energy can lead to significant deviations in the predicted expiration date.
Distinguishing between reaction types is crucial for selecting the correct model.
- N-th order reactions proceed at a rate proportional to the concentration of the remaining reactant.
- Autocatalytic reactions accelerate as products are formed, often leading to sudden failure after an induction period.
- Solid-state reactions are governed by nucleation and growth mechanisms distinct from liquid-phase kinetics.
Adherence to standard test methods ensures data reliability and regulatory acceptance. ASTM E698 serves as the standard test method for determining Arrhenius kinetic constants for thermally unstable materials, primarily applicable to single-step, first-order reactions. Following such established protocols allows laboratories to defend their shelf-life claims during regulatory audits.
Using differential scanning calorimetry for stability assessment
Differential Scanning Calorimetry (DSC) is a primary tool for assessing the physical stability of materials. This technique measures the heat flow difference between a sample and a reference as they are heated, cooled, or held at a constant temperature. Changes in heat flow indicate phase transitions that are directly linked to product stability and shelf life.
The glass transition temperature (Tg) is a critical parameter for amorphous materials. Below the Tg, the material remains in a glassy, rigid state where molecular mobility is low, and chemical degradation is slow. Above the Tg, the material enters a rubbery state with increased molecular mobility, significantly accelerating degradation reactions.
Physical aging, or enthalpy relaxation, is another phenomenon monitored by DSC. Even below the Tg, amorphous materials slowly relax toward a lower energy equilibrium state, which can affect disintegration times and mechanical properties. DSC can quantify this relaxation, providing insight into the material's thermal history and potential for physical changes during storage.
Detecting the onset of crystallization is vital for lyophilized pharmaceutical products.
- Amorphous drugs typically have higher solubility but lower stability than crystalline forms.
- Crystallization can lead to reduced efficacy and changes in dissolution rates.
- DSC scans reveal the crystallization exotherm, indicating the temperature limit for stable storage.
Protein stability analysis also relies heavily on DSC data. The technique measures the thermal denaturation temperature (Tm), which marks the point where the protein structure unfolds. A higher Tm correlates with greater conformational stability, suggesting a longer shelf life under standard storage conditions.
Advanced DSC techniques, such as Modulated DSC (MDSC), separate reversible and non-reversible heat flow events. This separation allows analysts to accurately measure weak glass transitions that might overlap with other thermal events. MDSC provides the resolution needed to detect subtle instability markers in complex formulations.
In MDSC, the "reversing" signal typically captures heat capacity-related events like the glass transition. The "non-reversing" signal captures kinetic events such as crystallization, curing, or decomposition. Distinguishing these signals enables a more accurate interpretation of stability-indicating profiles in multi-component mixtures.
Applying thermogravimetric analysis for decomposition kinetics
Thermogravimetric Analysis (TGA) measures the change in sample mass as a function of temperature or time. This technique is essential for determining the thermal stability of materials that undergo decomposition, oxidation, or desolvation. By monitoring mass loss, laboratory professionals can pinpoint the exact onset of thermal degradation.
TGA kinetics are particularly useful for estimating the lifetime of polymers and inorganic materials. The method involves running samples at multiple heating rates (e.g., 2, 5, 10, and 20 °C/min) to shift the decomposition profile. The shift in decomposition temperature with heating rate provides the data necessary to calculate activation energy using the Doyle or Flynn-Wall-Ozawa methods.
ASTM E1641 describes the standard test method for decomposition kinetics by thermogravimetry. This standard provides a rigorous framework for determining the kinetic parameters required for lifetime prediction. Analysts use these parameters to generate a lifetime plot, which estimates the time to failure at any given use temperature.
Key applications of TGA in shelf-life modeling include:
- Solvent evaporation: Quantifying residual solvents that may catalyze degradation.
- Oxidative stability: Measuring the oxygen uptake onset temperature (OOT).
- Composite degradation: Determining the thermal endurance of packaging materials.
Beyond dynamic heating, TGA is used to determine Oxidative Induction Time (OIT). In an OIT experiment, the sample is held isothermally at an elevated temperature under an oxygen atmosphere to measure the time until oxidation begins. This specific parameter is widely used to predict the longevity of polyolefins and oils under oxidative stress.
Coupling TGA with evolved gas analysis (EGA) techniques, such as mass spectrometry, enhances predictive capabilities. This combination identifies the specific gaseous byproducts evolved during decomposition. Understanding the chemical nature of the breakdown products helps analysts elucidate the degradation pathway and design more stable formulations.
Selection of the purge gas is a critical experimental variable in TGA.
- Nitrogen creates an inert atmosphere to study purely thermal decomposition.
- Air or Oxygen is used to study oxidative degradation, which often occurs at lower temperatures.
- Variable atmospheres can simulate specific storage environments or processing conditions.
Evaluating the impact of moisture and humidity on shelf life
Moisture content is a dominant factor in the shelf-life reduction of hygroscopic materials. Water acts as a plasticizer, lowering the glass transition temperature and increasing molecular mobility. This plasticization effect facilitates chemical reactivity and physical instability, such as caking or crystallization.
Thermal analysis for shelf-life prediction often incorporates data from Dynamic Vapor Sorption (DVS) or TGA-Sorption systems. These instruments measure the mass change of a sample as it is exposed to controlled relative humidity (RH) profiles. The resulting sorption-desorption isotherms reveal the critical relative humidity (CRH) at which the material typically becomes unstable.
Water activity (aw) is often more predictive of stability than total moisture content.
- Microbial growth is directly linked to available water measured by aw.
- Chemical reaction rates (e.g., hydrolysis) often correlate exponentially with aw.
- Sorption isotherms map the relationship between moisture content and aw at a specific temperature.
Understanding the interaction between temperature and humidity is essential for accurate modeling. The modified Arrhenius equation addresses moisture sensitivity by including a humidity term. This expanded model predicts stability under real-world storage conditions where both temperature and humidity fluctuate.
Strategies for mitigating moisture-induced instability involve formulation adjustments.
- Excipient selection: Using non-hygroscopic fillers to buffer moisture uptake.
- Packaging barriers: Selecting materials with low water vapor transmission rates.
- Desiccants: Incorporating drying agents to maintain low humidity within the package.
Monitoring the Tg as a function of moisture content allows for the construction of a state diagram. This diagram maps the stable and unstable regions of a product based on temperature and water content. Laboratory professionals use state diagrams to define safe storage and handling envelopes for sensitive products.
Achieving regulatory compliance and kinetic software validation
Regulatory bodies demand rigorous validation of the methods used for shelf-life prediction. The FDA and international agencies recognize thermal analysis as a supportive tool for stability testing, provided the data is robust and the models are validated. The International Conference on Harmonisation (ICH) Q1A(R2) guideline outlines the requirements for stability testing of new drug substances and products.
In addition to Q1A, the ICH Q1E guideline provides recommendations on the evaluation of stability data. It allows limited extrapolation of shelf life beyond available real-time data under justified and well-controlled conditions. Thermal analysis data often serves as the scientific justification for these extrapolations during early development phases.
Advanced kinetic software is essential for processing the complex data generated by DSC and TGA. This software automates the calculation of activation energy, pre-exponential factors, and reaction order. Using validated software reduces human error and ensures that calculations comply with standard methods like ASTM E698 and ASTM E1641.
Software features that support compliance include:
- Audit trails: Tracking all data manipulation and parameter changes.
- User access controls: Restricting method modification to authorized personnel.
- Data integrity checks: Ensuring raw data files remain unaltered and traceable.
For electronic records, compliance with 21 CFR Part 11 is mandatory. Laboratory software must ensure that electronic signatures are secure and that records cannot be deleted or modified without detection. Validators must verify that the kinetic software performs calculations accurately by testing it against known data sets.
While accelerated predictive modeling is powerful, it does not entirely replace real-time stability testing. Regulatory submissions typically require long-term stability data to confirm the predictions made by thermal analysis. However, predictive modeling significantly de-risks the development process by ensuring that only the most stable formulations proceed to long-term trials.
Verification of the predictive model is a continuous process. As real-time stability data becomes available, analysts compare it against the predicted values. Discrepancies lead to refinement of the kinetic model, improving the accuracy of future predictions for similar product lines.
Summary of thermal analysis for shelf-life prediction
Thermal analysis for shelf-life prediction offers a scientifically grounded method for estimating product stability and expiration dates. By utilizing techniques such as DSC and TGA, laboratory professionals can determine critical kinetic parameters like activation energy and glass transition temperatures. These metrics allow for the rapid screening of formulations and the construction of accurate degradation models. Integrating these analytical tools with compliant software and adherence to standards like ASTM E698 ensures that stability claims are both reliable and regulatory-ready. Ultimately, thermal analysis accelerates the development cycle while maintaining high standards of product quality and safety.
This article was created with the assistance of Generative AI and has undergone editorial review before publishing.













